| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Original Article
Volume 17, Number 1, February 2024, pages 23-31
Clinicopathological Aspects and Inflammation-Immune Markers in Alcohol and/or Hepatitis C Virus-Induced Hepatocellular Carcinoma Patients Treated With Sorafenib
Thiago Alexandre Martins Pintoa, Helena Paes Almeida Saitoa, Carolina Lopes Nourania, Elaine Cristina Ataideb, Ilka Fatima Santana Ferreira Boinb, Gustavo Jacob Lourencoc, Carmen Silvia Passos Limaa, c, d
aClinical Oncology Service, Department of Anesthesiology, Oncology, and Radiology, School of Medical Sciences, University of Campinas, Campinas, Sao Paulo, Brazil
bDepartment of Surgery, School of Medical Sciences, University of Campinas, Campinas, Sao Paulo, Brazil
cLaboratory of Cancer Genetics; School of Medical Sciences, University of Campinas, Campinas, Sao Paulo, Brazil
dCorresponding Author: Carmen Silvia Passos Lima, Clinical Oncology Service, Department of Anesthesiology, Oncology, and Radiology, School of Medical Sciences, University of Campinas, Rua Alexander Fleming, 181, Cidade Universitaria “Zeferino Vaz”, Barao Geraldo, Campinas, Sao Paulo, Brazil
Manuscript submitted November 30, 2023, accepted January 6, 2024, published online February 28, 2024
Short title: AFP Level and Child-Pugh Score in HCC Prognosis
doi: https://doi.org/10.14740/gr1689
| Abstract | ▴Top |
Background: Tyrosine kinase inhibitors have been used to treat hepatocellular carcinoma (HCC), but the outcomes of patients under treatment vary. Since the roles of clinicopathological aspects and markers of chronic inflammation/immune homeostasis in the outcome of HCC patients treated with sorafenib are still unclear, these were the aims of this study.
Methods: Patients with alcohol-induced and/or hepatitis C virus (HCV)-induced HCC (n = 182) uniformly treated with sorafenib were included in the study. Baseline clinicopathological aspects of patients were computed from the medical records. The neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), lymphocyte-to-monocyte ratio (LMR), systemic inflammation response index (SIRI), and systemic immune-inflammation index (SII) were obtained from the hematological exam performed before the administration of sorafenib. Overall survival (OS) was analyzed using Kaplan-Meier probabilities, log-rank test, and univariate and multivariate Cox proportional hazard ratio (HR) analyses.
Results: In multivariate analysis, alpha-foetoprotein (AFP) level and Child-Pugh score were predictors of OS. Patients with AFP levels higher than 157 ng/mL and Child-Pugh B or C had 1.40 (95% confidence interval (CI): 1.03 - 1.91, P = 0.03) and 1.64 (95% CI: 1.07 - 2.52, P = 0.02) more chances of evolving to death than the remaining patients, respectively. NLR, PLR, LMR, SIRI, and SII did not alter the OS of HCC patients.
Conclusions: AFP level and Child-Pugh score act as independent prognostic factors in patients with alcohol and/or HCV-induced HCC treated with sorafenib, but markers of chronic inflammation/immune homeostasis seem not to alter the outcome of patients with HCC induced by alcohol and/or HCV.
Keywords: Hepatocellular carcinoma; Lymphocyte-to-monocyte ratio; Neutrophil-to-lymphocyte ratio; Platelet-to-lymphocyte ratio; Prognosis; SIRI; SII; Sorafenib
| Introduction | ▴Top |
Hepatocellular carcinoma (HCC) accounts for about 5% of all cancer cases, but it is the fourth leading cause of death from cancer worldwide [1]. The 5-year overall survival (OS) rate for HCC patients stands below 20% [2]. The increasing number of cases in Brazil and the apparent intense aggressiveness of the tumor in the country make it a relevant pathology for research [3, 4].
Curative treatments, such as surgical resection and radiofrequency ablation, are indicated for patients with early-stage tumors [5, 6]. Nevertheless, most patients with HCC are diagnosed at intermediate or advanced stages, and these therapies are not effective for them [5, 6]. Sorafenib, a multikinase inhibitor that blocks cell proliferation, is the only first-line systemic therapy that improves OS of unresectable HCC patients [7, 8], but the results presented by different patients under treatment vary.
It was already postulated that clinicopathological aspects, such as age [9], performance status [9-13], non-viral etiologies [9], Child-Pugh score [10-17], alpha-foetoprotein (AFP) level [13, 14, 16, 18, 19], type 2 diabetes mellitus (DM2) [19, 20], sarcopenia [21], and tumor size/volume [18, 19] are independent prognostic factors for HCC patients treated with sorafenib, but most of these associations need to be confirmed.
Chronic inflammation has been seen as a typical feature of HCC, being present in approximately 90% of cases [22, 23], and increasing evidence shows that systemic inflammation markers correlate with the HCC patients’ outcomes. The neutrophil-to-lymphocyte ratio (NLR) [12, 15, 21, 24, 25] and platelet-to-lymphocyte ratio (PLR) [9, 12, 15, 21, 24, 25] effects on OS of patients with HCC of various etiologies undergoing sorafenib therapy or combinations of tyrosine kinase inhibitor (TKI) and checkpoint inhibitors therapy were investigated, and conflicting results were obtained.
The immune system has been implicated in the origin, progression, and outcome of HCC patients [26, 27]. It has also been seen with potential importance in sorafenib treatment [28-30], giving support to investigations of baseline lymphocyte-to-monocyte ratio (LMR) in HCC patients treated with the agent. Conflicting results were obtained in the evaluation of the LMR effects on survival of patients with HCC of viral and non-viral etiology treated with sorafenib or combinations of checkpoint inhibitors and TKIs [13, 15, 16, 21].
Conflicting results were also found in analysis of the systemic inflammation response index (SIRI) in OS of patients with HCC induced by hepatitis B virus (HBV) infection treated with sorafenib [16] or combinations of checkpoint inhibitors and TKIs [21] and of the systemic inflammatory index (SII) in OS of patients with viral and non-viral HCC [9, 15, 24] receiving sorafenib.
In the current study, we analyzed the effects of baseline clinicopathological aspects and inflammation-immune markers in HCC induced mainly by alcohol and hepatitis C virus (HCV) uniformly treated with sorafenib, aiming to assess their roles in patients’ outcomes.
| Materials and Methods | ▴Top |
Study design and participants
All HCC patients treated with sorafenib at the University of Campinas’s General Hospital between July 2010 and March 2020 were enrolled in this retrospective study. HCC diagnosis was based on conventional criteria [31]. Patients with inconsistent follow-up data, or those whose data could not be obtained, were excluded from the analysis.
Data related to age, gender, race, alcohol or tobacco use, body mass index (BMI), infection by HBV or HCV, and the presence of DM2 as a comorbidity of interest were collected from the medical records. Obesity [32] and DM2 [33] were identified using conventional criteria, and Child-Pugh staging was based on the Barcelona Clinic Liver Cancer (BCLC) system. A hematological exam and AFP level were obtained before sorafenib initiation. SIRI and SII were calculated as follows: SIRI = peripheral blood neutrophil count × monocyte/lymphocyte count and SII = peripheral blood platelet count × neutrophil count/lymphocyte count obtained prior to sorafenib administration. Median values were used as cutoff values for NLR, PLR, LMR, SIRI, and SII.
All patients were treated with oral sorafenib at an initial daily dose of 800 mg [7]. During treatment, patients were queried regarding adverse events, and had reductions in agent dose or treatment interruption, based on the National Cancer Institute’s criteria (CTCAE 2017).
Statistical analysis
The statistical significance of differences between groups was calculated either using Fisher’s exact probability test or the Chi-square test and multiple logistic regression. OS was calculated from the date of diagnosis until the date of death from any cause or the date of the last follow-up, using Kaplan-Meier estimated probability curves and the log-rank test. The impact of clinicopathological aspects on patients’ survival was assessed through univariate and multivariate Cox analyses; factors with P < 0.10 were accepted for multivariate analysis. A bootstrap analysis (n = 10,000) based on random resampling was applied to ensure the stability of the Cox regression model.
All analyses were performed with the SPSS 21.0 statistical program (SPSS Incorporation, USA). Factors with P values < 0.05 were considered significant.
The study was conducted in accordance with the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the research ethics committees of the University of Campinas (process no 4.139.074).
| Results | ▴Top |
Description of the cohort
The study revealed that the median age of the 182 patients enrolled in the study was 60 years, and most of them were males. About two-thirds of the patients were alcoholics or former alcoholics and had hepatitis C, half of the patients were smokers or former smokers, and about one-third of the patients had DM2. Almost all patients were categorized as Child-Pugh A or B (Table 1).
 Click to view | Table 1. Baseline Characteristics of the 182 Patients With Hepatocellular Carcinoma |
Grade 3 toxicities to sorafenib identified in 35 (19.2%) patients were: diarrhea (14 patients), hand-foot syndrome (10 patients), nausea (four patients), asthenia (three patients), myalgia (two patients), thrombocytopenia (one patient), and skin lesion (one patient) (Table 1).
Patient survival
The median observation time of patients in the study was 20 months (range = 1 - 143), and at this time, 50.5% of patients were alive and 49.5% had died (Fig. 1). At the end of the study (10/03/2023), five patients (2.7%) were alive and 177 (97.3%) were dead.
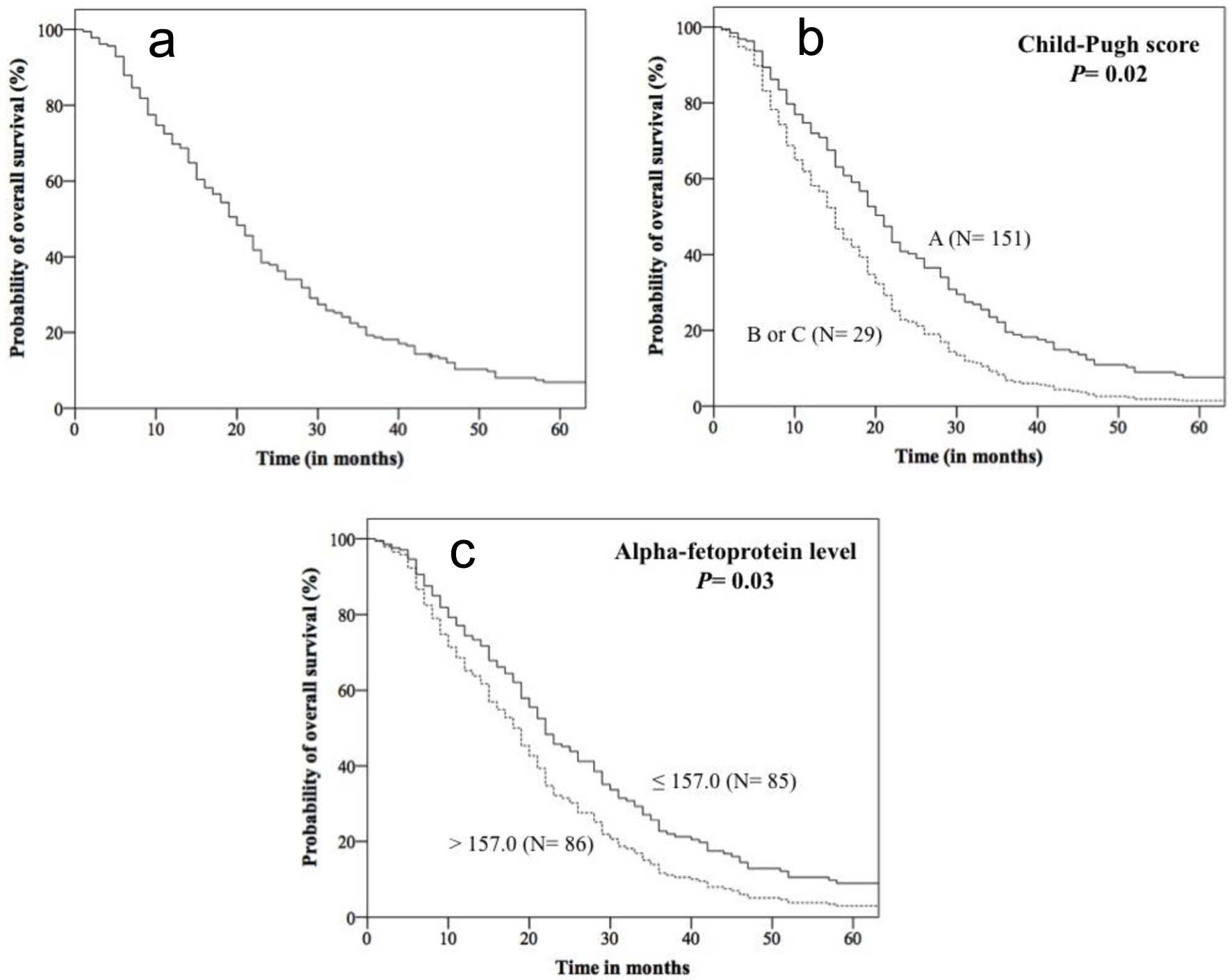 Click for large image | Figure 1. Kaplan-Meier curves for overall survival of 182 patients with hepatocellular carcinoma treated with sorafenib obtained from data of Cox’s multivariate analysis. Overall survival of the entire cohort (a), and of patients stratified by Child-Pugh classification (b), and alpha-fetoprotein level (c). |
At 20 months of follow-up, similar OS rates were observed in patients stratified by age, gender, alcohol consumption, smoking habit, obesity, HBV status and HCV status, NLR, PLR, LMR, SIRI, and SII. In contrast, lower OS was observed in patients with DM2 (40.0% vs. 54.1%, P = 0.02), AFP > 157.0 ng/mL (46.9% vs. 62.7%, P = 0.04), and Child-Pugh B or C (41.4% vs. 52.3%, P = 0.007), when compared to others (Kaplan-Meier estimates). In univariate analysis, patients with DM2, AFP > 157.0 ng/mL, and Child-Pugh B or C had a 1.42 (95% confidence interval (CI): 1.03 - 1.94), 1.34 (95% CI: 1.00 - 1.82) and 1.72 (95% CI: 1.14 - 2.59) more chances of evolving to death than the remaining patients, respectively.
In multivariate analysis, patients with AFP levels higher than 157.0 ng/mL and Child-Pugh B or C had 1.40 (95% CI: 1.03 - 1.91) and 1.64 (95% CI: 1.07 - 2.52) more chances of dying than the remaining patients, respectively (Fig. 1, Table 2).
 Click to view | Table 2. Association of Clinical and Pathological Aspects With Overall Survival of 182 Patients With Hepatocellular Carcinoma |
| Discussion | ▴Top |
We initially found that HCC patients enrolled in this study generally presented a clinicopathological profile like those of patients from other countries [34], and therefore the sample analyzed herein was adequate for the evaluation of prognostic factors in the disease. However, it is important to note that most patients in our sample had alcohol and HCV-induced HCC, while chronic infection by HBV was the main cause of HCC among European and Asian patients [34]. Moreover, patients with Child-Pugh A or B were more prevalent in our sample than in others [34], because only cases with unequivocal indication for sorafenib treatment were treated in our service.
Secondly, Cox’s univariate analysis revealed that the presence of DM2, AFP level higher than 157 ng/mL, and Child-Pugh B or C were associated with increased risk of death in our sample; therefore, these markers were seen as prognostic predictors for OS. Lower OS was also observed in patients with AFP levels higher than 157 ng/mL and in patients with Child-Pugh B or C in Cox’s multivariate analysis, even after Bonferroni correction, and therefore these markers were seen as independent predictive factors for OS in our patients.
DM predicted a better prognosis in HCC treated with sorafenib in two studies [19, 20]. Since hyperglycemia favors glycolysis which is a main energy-producing process in cancer cells [35], the authors attributed the association of DM2 with longer survival of HCC patients treated with sorafenib to the effects of TKIs in glycemic control in diabetics reducing proliferation of the malignant cells. DM2 had a mildly negative impact on our patients’ survival in univariate analysis, but this result was not confirmed in multivariate analysis. It is possible that the survival of patients with DM2 was altered by other risk factors whose influences were excluded in Cox’s univariate analysis. In accordance with our finding, no survival difference between diabetic and nondiabetic HCC patients treated with sorafenib was seen in other two studies [11, 36], and therefore the association of DM2 with the outcome of HCC patients treated with sorafenib remains controversial in literature.
The associations of high AFP level and Child-Pugh B or C with low OS in our patients were not a surprise. High AFP level was previously associated with low survival rates in patients with HCC induced mainly by HBV and/or HCV treated with sorafenib [13, 14, 16, 18, 19]. Previous studies have shown that the time required for the tumor to double in volume, as well as the time for AFP levels to double, were shorter in HCC with higher degrees of malignancy [37]. Moreover, having a good hepatic reserve is the main variable in defining OS in patients with alcohol, HBV and/or HCV-induced HCC undergoing sorafenib therapy. It is well known that Child-Pugh A or B patients obtain the best therapeutic results [10-13, 15-17].
Finally, we did not find associations of markers and indexes of chronic inflammation/immune homeostasis with the outcome of patients with HCC patients’ outcome in the current study. Neutrophils promote adhesion and seeding of tumor cells in distant organ sites through the secretion of growth factors and proteases [38], and mechanically, elevated neutrophils reflect the response to the system inflammation that is related to the increasing tumor burden and metastasis [39]. Platelets induce circulating tumor cell epithelial-mesenchymal transition and promote tumor metastasis [40, 41]. Monocytes/macrophages in the tumor act as a pro-cancer microenvironment by facilitating tumor cell migration, invasion, and metastasis, and by protecting the tumor from the anti-tumor immune response [42-44]. In contrast, lymphocytes play a fundamental role in host defense against tumors by inducing cytotoxic cell death and inhibiting tumor cell proliferation and migration [45]. Thus, the lack of association between patients’ OS and inflammation/immune homeostasis markers and indexes was not expected in the current study.
In fact, elevated NLR and SII predicted lower OS in HCC patients in the study conducted by Casadei Gardini et al [24] and Conroy et al [15] showed high SII as a risk factor for poor OS in HCC patients. Zhu et al [16] observed that elevated monocyte-to-lymphocyte ratio predicted low OS in HCC patients. Ha et al [13] in an analysis of advanced HCC in two ethnically distinct groups of HCC patients, North Americans, and Asians, observed that reduced LMR predicted low OS only in the Asian subset. Elevated NLR and low PLR were also predictive factors of low OS benefit in HCC patients in the meta-analysis of 13 studies conducted by Liu et al [25]. Elevated SII predicted low OS in patients with HCC in the study conducted by Zhao et al [21]. It is worth commenting that HCC was induced mainly by HBV and HCV [13, 16, 21, 25] and that HCC patients received only sorafenib as treatment [13, 15, 16, 24, 25] in most of the above-mentioned studies. A substantial cohort of patients with HCC induced by various chronic liver diseases was included only in studies conducted by Casadei Gardini et al (viral etiology: 54%, non-viral etiology: 44%) and Conroy et al (viral etiology: 30%, alcohol: 50%, other etiologies: 20%), and treatment with various combinations of TKIs and immune checkpoint inhibitors (ICIs) was administered only to HCC patients enrolled in the study of Zhao et al. Nevertheless, OS was not altered by PLR in Casadei Gardini’s study, NLR, PLR, and LMR in Conroy’s study, PLR and SII in Rovesty’s study, NLR and PLR in Sprinzl’s study, SIRI in Zhu’s study, and NLR, PLR, and LMR in Zhao’s study. It is also important to highlight at this point in the discussion that non-virus-related HCC contributed substantially to the cohorts analyzed in the studies conducted by Casadei Gardini et al, Conroy et al and Sprinzl et al (viral etiology: 40%, alcohol: 30%, other etiologies: 30%), and that sorafenib was the unique systemic treatment administered to patients enrolled in all studies, except in the study of Zhao et al.
It has been reported that alcohol accelerates HCV-induced liver tumorigenesis [46], and patients with HCC induced by alcohol abuse and steatohepatitis of other etiologies have poorer prognosis than those with virus-induced HCC [9], possibly because they have not been subjected to HCC screening and therefore have been diagnosed with advanced stage HCC with worse liver function [47]. Sorafenib may have distinct antitumor effects in patients with HCC induced by HBV or HCV [48]. Moreover, lymphocytes are more common in ascitic fluid [49], peripheral blood, and liver tissue [50] of patients with cirrhosis/HCC of viral etiology (HBV or HCV) than in the respective fluids and tissue of patients with cirrhosis/HCC of other etiologies, including alcohol. It is possible that differences in HCC etiologies may influence the association of inflammation/immune homeostasis markers and indexes with OS. Approximately 60% of patients enrolled in the current study had HCC induced by alcohol and/or HCV. Thus, our data and data of Casadei Gardini et al, Conroy et al, and Sprinzl et al conducted in patients with viral HCC, non-viral HCC, or HCC of mixed etiology, suggest that inflammation/immune homeostasis markers and indexes may not predict OS in this group of patients. Differences in sample size, treatments, and follow-up time may also explain the controversial data found in the studies. It is possible that further studies focusing on patients with HCC of the same etiology and homogeneously treated with sorafenib are needed to clarify this issue.
We are aware of limitations of the present study. The medium-sized sample, a single-center service, the retrospective nature of the study, and the lack of additional information about clinical and tumor aspects, such as sarcopenia and tumor size/volume, could act as confounding factors in the analysis of survival rates. Moreover, analysis of inflammatory and immune cells in the tumor, not performed in the study, would also be important to clarify whether the composition of these cells in peripheral blood reflects their composition in the HCC microenvironment.
In summary, our data indicate that AFP level and Child-Pugh score act as independent prognostic factors in patients with alcohol and/or HCV-induced HCC treated with sorafenib, and similar results were previously described in patients with HCC of various etiologies [10-13, 15-19]. Nevertheless, our data and data of previous studies conducted in HCC induced by viral HCC, non-viral HCC, or HCC of mixed etiology [9, 12, 15, 16, 24] undergoing sorafenib therapy do not show associations of markers of chronic inflammation/immune homeostasis with patients’ survival. We present chronic inflammation/immune homeostasis markers as unimportant prognostic factors in patients with alcohol and/or HCV-induced HCC treated with sorafenib, and this is the novel finding of the study. We believe that if these findings could be confirmed in a larger cohort of patients with better clinical and tumor characterization, they could provide valuable information to health care professionals, who should consider AFP level and Child-Pugh score, but not inflammation/immune homeostasis markers and indexes as seen in HCC of other etiologies to predict OS in this group of patients.
Acknowledgments
None to declare.
Financial Disclosure
This research was not funded externally.
Conflict of Interest
All authors declare that they have no conflict of interest.
Informed Consent
Written informed consent was obtained from patients or their guardians.
Author Contributions
Thiago Alexandre Martins Pinto: data analysis, data interpretation, manuscript preparation and manuscript editing; Helena Paes Almeida Saito: clinical assistance to patients and data acquisition; Carolina Lopes Nourani: clinical assistance to patients and data acquisition; Elaine Cristina Ataide: clinical assistance to patients and data acquisition; Ilka Fatima Santana Ferreira Boin: data acquisition, quality control of data and algorithms and data interpretation; Gustavo Jacob Lourenco: statistical analysis, data interpretation and manuscript preparation; and Carmen Silvia Passos Lima: study concepts, quality control of data and algorithms, interpretation, and manuscript editing. All authors participated and approved the final version of the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article. Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
Abbreviations
HCC: hepatocellular carcinoma; OS: overall survival; AFP: alpha-foetoprotein; DM2: type 2 diabetes mellitus; NLR: neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio; LMR: lymphocyte-to-monocyte ratio; SIRI: systemic inflammation response index; SII: systemic immune-inflammation index; HCV: hepatis C virus; HBV: hepatitis B virus
| References | ▴Top |
- Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Pineros M, Znaor A, Bray F. Cancer statistics for the year 2020: An overview. Int J Cancer. 2021;149:778-789.
doi pubmed - Global Cancer Facts & Figures, 3rd Edition. Available from: http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-044738.pdf.
- Carrilho FJ, Kikuchi L, Branco F, Goncalves CS, Mattos AA, Brazilian HCCSG. Clinical and epidemiological aspects of hepatocellular carcinoma in Brazil. Clinics (Sao Paulo). 2010;65(12):1285-1290.
doi pubmed pmc - Kikuchi L, Chagas AL, Alencar RS, Paranagua-Vezozzo DC, Carrilho FJ. Clinical and epidemiological aspects of hepatocellular carcinoma in Brazil. Antivir Ther. 2013;18(3 Pt B):445-449.
doi pubmed - Bruix J, Sherman M, American Association for the Study of Liver D. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020-1022.
doi pubmed pmc - European Association for the Study of the Liver-European Organization for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908-943.
doi pubmed - Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378-390.
doi pubmed - Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25-34.
doi pubmed - Rovesti G, Orsi G, Kalliopi A, Vivaldi C, Marisi G, Faloppi L, Foschi FG, et al. Impact of baseline characteristics on the overall survival of HCC patients treated with sorafenib: ten years of experience. Gastrointest Tumors. 2019;6(3-4):92-107.
doi pubmed pmc - Diaz-Beveridge R, Bruixola G, Lorente D, Caballero J, Rodrigo E, Segura A, Akhoundova D, et al. An internally validated new clinical and inflammation-based prognostic score for patients with advanced hepatocellular carcinoma treated with sorafenib. Clin Transl Oncol. 2018;20(3):322-329.
doi pubmed - Labenz C, Prenosil V, Koch S, Huber Y, Marquardt JU, Schattenberg JM, Galle PR, et al. Impact of individual components of the metabolic syndrome on the outcome of patients with advanced hepatocellular carcinoma treated with sorafenib. Dig Dis. 2018;36(1):78-88.
doi pubmed - Sprinzl MF, Kirstein MM, Koch S, Seib ML, Weinmann-Menke J, Lang H, Duber C, et al. Improved prediction of survival by a risk factor-integrating inflammatory score in sorafenib-treated hepatocellular carcinoma. Liver Cancer. 2019;8(5):387-402.
doi pubmed pmc - Ha Y, Mohamed Ali MA, Petersen MM, Harmsen WS, Therneau TM, Lee HC, Ryoo BY, et al. Lymphocyte to monocyte ratio-based nomogram for predicting outcomes of hepatocellular carcinoma treated with sorafenib. Hepatol Int. 2020;14(5):776-787.
doi pubmed pmc - da Fonseca LG, Barroso-Sousa R, Bento Ada S, Blanco BP, Valente GL, Pfiffer TE, Hoff PM, et al. Pre-treatment neutrophil-to-lymphocyte ratio affects survival in patients with advanced hepatocellular carcinoma treated with sorafenib. Med Oncol. 2014;31(11):264.
doi pubmed - Conroy G, Salleron J, Belle A, Bensenane M, Nani A, Ayav A, Peiffert D, et al. The prognostic value of inflammation-based scores in advanced hepatocellular carcinoma patients prior to treatment with sorafenib. Oncotarget. 2017;8(56):95853-95864.
doi pubmed pmc - Zhu Z, Xu L, Zhuang L, Ning Z, Zhang C, Yan X, Lin J, et al. Role of monocyte-to-lymphocyte ratio in predicting sorafenib response in patients with advanced hepatocellular carcinoma. Onco Targets Ther. 2018;11:6731-6740.
doi pubmed pmc - Labeur TA, Achterbergh R, Takkenberg B, Van Delden O, Mathot R, Klumpen HJ. Sorafenib for patients with hepatocellular carcinoma and Child-Pugh B liver cirrhosis: lessons learned from a terminated study. Oncologist. 2020;25(9):e1274-e1279.
doi pubmed pmc - Yuan J, Liang H, Li J, Li M, Tang B, Ma H, Xie X, et al. Peripheral blood neutrophil count as a prognostic factor for patients with hepatocellular carcinoma treated with sorafenib. Mol Clin Oncol. 2017;7(5):837-842.
doi pubmed pmc - Hsieh MH, Kao TY, Hsieh TH, Kao CC, Peng CY, Lai HC, Chuang PH, et al. Prognostic roles of diabetes mellitus and hypertension in advanced hepatocellular carcinoma treated with sorafenib. PLoS One. 2020;15(12):e0244293.
doi pubmed pmc - Di Costanzo GG, Tortora R, Morisco F, Addario L, Guarino M, Cordone G, Falco L, et al. Impact of diabetes on outcomes of sorafenib therapy for hepatocellular carcinoma. Target Oncol. 2017;12(1):61-67.
doi pubmed - Zhao M, Duan X, Han X, Wang J, Han G, Mi L, Shi J, et al. Sarcopenia and systemic inflammation response index predict response to systemic therapy for hepatocellular carcinoma and are associated with immune cells. Front Oncol. 2022;12:854096.
doi pubmed pmc - Barash H, E RG, Edrei Y, Ella E, Israel A, Cohen I, Corchia N, et al. Accelerated carcinogenesis following liver regeneration is associated with chronic inflammation-induced double-strand DNA breaks. Proc Natl Acad Sci U S A. 2010;107(5):2207-2212.
doi pubmed pmc - Margetts J, Ogle LF, Chan SL, Chan AWH, Chan KCA, Jamieson D, Willoughby CE, et al. Neutrophils: driving progression and poor prognosis in hepatocellular carcinoma? Br J Cancer. 2018;118(2):248-257.
doi pubmed pmc - Casadei Gardini A, Scarpi E, Faloppi L, Scartozzi M, Silvestris N, Santini D, de Stefano G, et al. Immune inflammation indicators and implication for immune modulation strategies in advanced hepatocellular carcinoma patients receiving sorafenib. Oncotarget. 2016;7(41):67142-67149.
doi pubmed pmc - Liu L, Gong Y, Zhang Q, Cai P, Feng L. Prognostic roles of blood inflammatory markers in hepatocellular carcinoma patients taking sorafenib. a systematic review and meta-analysis. Front Oncol. 2019;9:1557.
doi pubmed pmc - Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331(6024):1565-1570.
doi pubmed - Chen KJ, Zhou L, Xie HY, Ahmed TE, Feng XW, Zheng SS. Intratumoral regulatory T cells alone or in combination with cytotoxic T cells predict prognosis of hepatocellular carcinoma after resection. Med Oncol. 2012;29(3):1817-1826.
doi pubmed - Hipp MM, Hilf N, Walter S, Werth D, Brauer KM, Radsak MP, Weinschenk T, et al. Sorafenib, but not sunitinib, affects function of dendritic cells and induction of primary immune responses. Blood. 2008;111(12):5610-5620.
doi pubmed - Cabrera R, Ararat M, Xu Y, Brusko T, Wasserfall C, Atkinson MA, Chang LJ, et al. Immune modulation of effector CD4+ and regulatory T cell function by sorafenib in patients with hepatocellular carcinoma. Cancer Immunol Immunother. 2013;62(4):737-746.
doi pubmed pmc - Flecken T, Schmidt N, Hild S, Gostick E, Drognitz O, Zeiser R, Schemmer P, et al. Immunodominance and functional alterations of tumor-associated antigen-specific CD8+ T-cell responses in hepatocellular carcinoma. Hepatology. 2014;59(4):1415-1426.
doi pubmed pmc - Ayuso C, Rimola J, Vilana R, Burrel M, Darnell A, Garcia-Criado A, Bianchi L, et al. Diagnosis and staging of hepatocellular carcinoma (HCC): current guidelines. Eur J Radiol. 2018;101:72-81.
doi pubmed - Bray GA, Heisel WE, Afshin A, Jensen MD, Dietz WH, Long M, Kushner RF, et al. The science of obesity management: an endocrine society scientific statement. Endocr Rev. 2018;39(2):79-132.
doi pubmed pmc - American Diabetes Association Professional Practice Committee. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S17-S38.
doi pubmed - Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589-604.
doi pubmed pmc - Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029-1033.
doi pubmed pmc - Casadei Gardini A, Faloppi L, De Matteis S, Foschi FG, Silvestris N, Tovoli F, Palmieri V, et al. Metformin and insulin impact on clinical outcome in patients with advanced hepatocellular carcinoma receiving sorafenib: Validation study and biological rationale. Eur J Cancer. 2017;86:106-114.
doi pubmed - Shingaki N, Tamai H, Mori Y, Moribata K, Enomoto S, Deguchi H, Ueda K, et al. Serological and histological indices of hepatocellular carcinoma and tumor volume doubling time. Mol Clin Oncol. 2013;1(6):977-981.
doi pubmed pmc - Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2015;12(12):681-700.
doi pubmed - Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646-674.
doi pubmed - Pages F, Galon J, Dieu-Nosjean MC, Tartour E, Sautes-Fridman C, Fridman WH. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. 2010;29(8):1093-1102.
doi pubmed - Bihari C, Rastogi A, Shasthry SM, Bajpai M, Bhadoria AS, Rajesh S, Mukund A, et al. Platelets contribute to growth and metastasis in hepatocellular carcinoma. APMIS. 2016;124(9):776-786.
doi pubmed - Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4(1):71-78.
doi pubmed - Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124(2):263-266.
doi pubmed - Kuang DM, Zhao Q, Peng C, Xu J, Zhang JP, Wu C, Zheng L. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med. 2009;206(6):1327-1337.
doi pubmed pmc - Mossanen JC, Tacke F. Role of lymphocytes in liver cancer. Oncoimmunology. 2013;2(11):e26468.
doi pubmed pmc - Matsushita H, Takaki A. Alcohol and hepatocellular carcinoma. BMJ Open Gastroenterol. 2019;6(1):e000260.
doi pubmed pmc - Ogasawara S, Chiba T, Ooka Y, Kanogawa N, Saito T, Motoyama T, Suzuki E, et al. Sorafenib treatment in Child-Pugh A and B patients with advanced hepatocellular carcinoma: safety, efficacy and prognostic factors. Invest New Drugs. 2015;33(3):729-739.
doi pubmed - Jackson R, Psarelli EE, Berhane S, Khan H, Johnson P. Impact of viral status on survival in patients receiving sorafenib for advanced hepatocellular cancer: a meta-analysis of randomized phase III trials. J Clin Oncol. 2017;35(6):622-628.
doi pubmed - Tapia-Abellan A, Martinez-Esparza M, Ruiz-Alcaraz AJ, Hernandez-Caselles T, Martinez-Pascual C, Miras-Lopez M, Such J, et al. The peritoneal macrophage inflammatory profile in cirrhosis depends on the alcoholic or hepatitis C viral etiology and is related to ERK phosphorylation. BMC Immunol. 2012;13:42.
doi pubmed pmc - Lim CJ, Lee YH, Pan L, Lai L, Chua C, Wasser M, Lim TKH, et al. Multidimensional analyses reveal distinct immune microenvironment in hepatitis B virus-related hepatocellular carcinoma. Gut. 2019;68(5):916-927.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


