| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Original Article
Volume 16, Number 1, February 2023, pages 25-36
The Impact of Metabolic Syndrome on the Prognosis of High-Risk Alcoholic Hepatitis Patients: Redefining Alcoholic Hepatitis
Shahid Habiba, f, Traci Murakamib, Varun Takyarc, Krunal Patela, Cristian Dominguezd, Yongcheng Zhane, Omid Mehrpoura, Chiu-Hsieh Hsue
aLiver Institute PLLC, Tucson, AZ, USA
bThe Queen’s Medical Center, West Oahu, HI, USA
cSutter Health, San Francisco, CA, USA
dDigestive Health Associates, Houston, TX, USA
eMel and Enid Zuckerman College of Public Health, University of Arizona, Tucson, AZ, USA
fCorresponding Author: Shahid Habib, Liver Institute PLLC, Tucson, AZ 85712, USA
Manuscript submitted July 11, 2022, accepted October 3, 2022, published online February 28, 2023
Short title: AH and IR Mortality Risk
doi: https://doi.org/10.14740/gr1556
| Abstract | ▴Top |
Background: Alcoholic hepatitis (AH) is characterized by acute symptomatic hepatitis associated with heavy alcohol use. This study was designed to assess the impact of metabolic syndrome on high-risk patients with AH with discriminant function (DF) score ≥ 32 and its effect on mortality.
Methods: We searched the hospital database for ICD-9 diagnosis codes of acute AH, alcoholic liver cirrhosis, and alcoholic liver damage. The entire cohort was categorized into two groups: AH and AH with metabolic syndrome. The effect of metabolic syndrome on mortality was evaluated. Also, an exploratory analysis was used to create a novel risk measure score to assess mortality.
Results: A large proportion (75.5%) of the patients identified in the database who had been treated as AH had other etiologies and did not meet the American College of Gastroenterology (ACG)-defined diagnosis of acute AH, thus had been misdiagnosed as AH. Such patients were excluded from analysis. The mean body mass index (BMI), hemoglobin (Hb), hematocrit (HCT), and alcoholic liver disease/non-alcoholic fatty liver disease index (ANI) were significantly different between two groups (P < 0.05). The results of a univariate Cox regression model showed that age, BMI, white blood cells (WBCs), creatinine (Cr), international normalized ratio (INR), prothrombin time (PT), albumin levels, albumin < 3.5, total bilirubin, Na, Child-Turcotte-Pugh (CTP), model for end-stage liver disease (MELD), MELD ≥ 21, MELD ≥ 18, DF score, and DF ≥ 32 had a significant effect on mortality. Patients with a MELD greater than 21 had a hazard ratio (HR) (95% confidence interval (CI) of 5.81 (2.74 - 12.30) (P < 0.001). The adjusted Cox regression model results showed that age, Hb, Cr, INR, Na, MELD score, DF score, and metabolic syndrome were independently associated with high patient mortality. However, the increase in BMI and mean corpuscular volume (MCV) and sodium significantly reduced the risk of death. We found that a model including age, MELD ≥ 21, and albumin < 3.5 was the best model in identifying patient mortality. Our study showed that patients admitted with a diagnosis of alcoholic liver disease with metabolic syndrome had an increased mortality risk compared to patients without metabolic syndrome, in high-risk patients with DF ≥ 32 and MELD ≥ 21. A bivariate correlation analysis revealed that patients with AH with metabolic syndrome were more likely to have infection (43%) compared to AH (26%) with correlation coefficient of 0.176 (P = 0.03, CI: 0.018 - 1.0).
Conclusion: In clinical practice, the diagnosis of AH is inaccurately applied. Metabolic syndrome significantly increases the mortality risk in high-risk AH. It signifies that the presence of features of metabolic syndrome modifies the behavior of AH in acute settings, warranting different therapeutic strategies. We propose that in defining AH, patients overlapping with metabolic syndrome may need to be excluded as their outcome is different with regard to risk of renal dysfunctions, infections and death.
Keywords: Alcoholic hepatitis; Metabolic syndrome; Discriminant function; MELD; Acute-on-chronic liver failure; Alcoholic liver disease; Alcoholic cirrhosis
| Introduction | ▴Top |
Alcoholic hepatitis (AH) is characterized by the onset of acute symptomatic hepatitis manifesting with features of liver failure in patients with stable alcoholic liver disease (ALD). AH could be the initial clinical presentation of ALD. Usually, it is associated with heavy alcohol use in preceding months. Most patients with AH have a history of alcohol use, usually more than 100 g/day for two or more decades [1, 2]. The incidence of AH is unknown because it often goes unreported. A cohort of 1,604 patients with a history of alcohol use found that about 20% had AH, suggesting that this disease is widespread [2]. AH is associated with the rapid onset or worsening of jaundice and, in severe cases, can lead to acute liver failure [3]. The mortality rate is as high as 16% and 30% at 1 and 3 months, respectively, with an overall 5-year survival rate of 56% [4].
In the United States, the burden of AH is increasing. In patients with severe AH (Maddrey discriminant function (DF) ≥ 32), short-term mortality rate is high at roughly 25-45% at 1 month [5-8]. The Maddrey DF is the most commonly used tool to assess the severity of AH and identify patients who may benefit from treatment with steroids, which has been debated over the years with inconsistent results [9]. This inconsistency could be due to a lack of a precise definition or diagnosis of AH or the presence of some other unidentified factors.
A transjugular liver biopsy is the best choice to diagnose patients with suspected AH. However, it is generally not performed in clinical practice. Currently, the diagnosis is based on a history of heavy alcohol consumption until within 3 months of index hospitalization and excluding other etiologies such as viral hepatitis (A, B, and C), autoimmune hepatitis, and Wilson’ disease [10].
Many prognostic models have been proposed to estimate the severity of AH, such as the MELD score [9] and Maddrey’s DF [11]. A DF value of 32 or higher indicates severe AH that carries an adverse prognosis with 20-30% mortality within 1 month after the presentation and 30-40% within 6 months after presentation [10].
The impact of metabolic syndrome on morbidity and mortality among patients consuming heavy alcohol and presenting with acute deterioration remains unknown, especially in high-risk patients with MELD ≥ 21 and DF ≥ 32. Lack of differentiation from metabolic syndrome could cause inconsistent results with steroid treatment and failure to develop new therapeutic modalities, such as extracorporeal liver assisted device (ELAD) [11, 12]. Obesity and metabolic syndrome are associated with increased liver-related mortality in stable ALD patients [13]. However, it is unclear whether hepatotoxic consequences of metabolic syndrome and alcohol intake are additive or synergistic [14]. Obesity and adipose tissue may contribute to the development of ALD by generating free radicals, increasing tumor necrosis factor-alpha (TNF-alpha) production, and producing fibrogenic agents [15]. On the other hand, alcohol causes hepatotoxicity through alcohol dehydrogenase, and cytochrome P450 2E1 (CYP2E1) pathways lead to toxic acetaldehyde production. In addition, CYP2E1 activates oxidative stress and pro-inflammatory TNF-alpha in the Kupffer cells [16]. Thus, in alcoholic and non-alcoholic steatohepatitis progression, there seem to be common metabolic aspects of the innate immune system and oxidative stress [17, 18].
To our knowledge, the impact of metabolic syndrome on AH has not been studied well. The presence of metabolic features may alter the disease course, including risk of acute deterioration and outcome. When such patients with combined alcohol and metabolic syndrome present with acute deterioration, their underlying etiologic and pathophysiologic mechanism of acute deterioration may not be the same as in patients with purely alcohol-related liver disease. In other words, two patients with a history of heavy alcoholism, one with metabolic syndrome and the other without, may have different mortality risks despite having high MELD or DF scores when they present with acute deterioration. Both may have different histopathological features despite the shared history of alcoholism; thus, they may need to be identified and treated as two separate and distinct entities for future clinical purposes. The impact of such factors has not been previously studied in the setting of acute AH.
This retrospective study aimed to assess factors associated with mortality risk in acute AH in high-risk patients (MELD ≥ 21 and DF ≥ 32). We hypothesize that the presence of insulin resistance manifesting with metabolic syndrome in such patients has a different outcome that could explain inconsistent response to treatment. Secondly, it aimed to identify factors associated with AH with metabolic syndrome to differentiate from patients with AH without metabolic syndrome. Thirdly, it aimed to assess the predictive ability of MELD ≥ 21 and DF ≥ 32 in conjunction with other factors such as age, infection, and albumin status.
| Materials and Methods | ▴Top |
We reviewed the hospital database for ICD-9 diagnosis codes of acute AH, alcoholic liver cirrhosis, and alcoholic liver damage over a period of 10 years. In addition, we retrospectively reviewed the charts of patients identified by database search for acute AH, alcoholic liver cirrhosis, and alcoholic liver damage, unspecified.
Inclusion and exclusion criteria were: 1) Age ≥ 18 years; 2) History of excess alcohol (as defined by alcohol use disorder criterion, > 5 drinks/day for males and > 4 drinks/day for females, at least one time per week) [19]; 3) Diagnosis of AH was based upon American College of Gastroenterology (ACG) guidelines as follows: patient with rapid development or worsening of jaundice and liver-related complications, with total serum bilirubin > 3 mg dL; alanine aminotransferase (ALT) and aspartate aminotransferase (AST) increased > 1.5 times the upper limit of normal, but less than 400 U/L with the AST/ALT ratio > 1.5; documentation of heavy alcohol consumption until 8 weeks before symptoms start [3]; 4) Exclusion of other etiologies (viral hepatitis A, B or C, autoimmune hepatitis, primary biliary cholangitis (PBC), or h/o known metabolic liver diseases, such as hemochromatosis, Wilson’s disease, or Alfa-1 anti-trypsin deficiency), biliary obstruction; 5) Exclusion of other conditions which may affect outcome such as hepatocellular carcinoma (HCC), extrahepatic malignancy, children, pregnant women, prisoners, or cognitively impaired adults.
The study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration. The University of Arizona Institutional Review Board (IRB) approved this study and waived the consent form requirement. Patients were identified through ICD-9 codes to diagnose AH, alcoholic cirrhosis, hepatitis, cirrhosis, and liver failure. Residents in Internal Medicine and Gastroenterology fellows reviewed the charts, and data were collected as per pre-defined protocol. Subsequently, the data were de-identified and submitted to a statistician for analysis. Figure 1 shows the steps of data processing and extraction.
 Click for large image | Figure 1. Data collection and analysis process. |
After identifying patients meeting inclusion and exclusion criteria, we reviewed all the charts for the entire course of hospital stay. We collected the following data: 1) demographic features including age, gender, height, weight, body mass index (BMI), race, and ethnicity; 2) history of alcohol drinking and other relevant social factors; 3) co-morbid conditions, especially features of metabolic syndrome; and 4) earliest (within 24 h of admission) laboratory values. Diagnosis of AH was based on the following clinical criteria defined by ACG as above metabolic syndrome was defined following the ATP-III guidelines (revised 2005), which consists of three or more of the following: fasting plasma glucose ≥ 100 mg/dL, serum triglycerides ≥ 150 mg/dL, serum high-density lipoprotein (HDL) cholesterol ≤ 40 mg/dL, blood pressure (BP) ≥ 130/85 mm Hg or BP medication or waist girths > 102 cm [20]. Because this study was retrospective, waist girth was not available; instead, we used BMI ≥ 30 as a criterion. Obesity classification was determined based on the World Health Organization (WHO) definitions: BMI ≥ 30 kg/m2 and < 30 [21]. Also, patients with A1c ≥ 5.7 or ≥ 6.3 were considered pre-diabetic or diabetic respectively; this was an alternative criterion for fasting blood glucose. We also calculated the ALD/non-alcoholic fatty liver disease (NAFLD) index (ANI) score, AST/ALT ratio, DF, AST to platelet ratio index (APRI), fibrosis-4 (Fib-4) score, MELD score, and Child-Turcotte-Pugh (CTP) classification at the time of admission.
DF score was defined as: 4.6 (Prothrombin time (s) - control Prothrombin time (s)) + serum bilirubin (mg/dL).
The MELD score was calculated using the formula: 3.8 (log bilirubin) + 11.2 (ln INR) + 9.6 (ln creatinine) + 10.
Infections, drug-induced liver injury (DILI) and gastrointestinal bleeding (GIB) are the known acute precipitants of a stable chronic liver disease leading to acute-on-chronic liver failure (ACLF) [22-24]. Infections were detected based on a positive culture (urine, blood, or ascites fluid), based on serological tests (cocci based on IgM), clinical examination (cellulitis), radiologist’s imaging report (pneumonia), or neutrophil granulocytes > 250 cells/µL (spontaneous bacterial peritonitis). The primary endpoint of the study was mortality. Mortality data were obtained from the Social Security Death Index.
Categorization
Patients were stratified into two groups based upon the presence or absence of metabolic syndrome, as shown in Figure 2. Patients who met inclusion and exclusion criteria, but with incomplete data were also excluded. A large proportion (75.5%) of the patients treated as AH had other etiologies as defined above or did not meet ACG-defined diagnosis of acute AH, thus had been misdiagnosed as AH. In a small proportion of patients (4%), either data were unavailable to assess metabolic syndrome features or complete labs were not available to calculate MELD and DF scores. Thus, only 20.5% of identified patients met the criteria to include for final analysis.
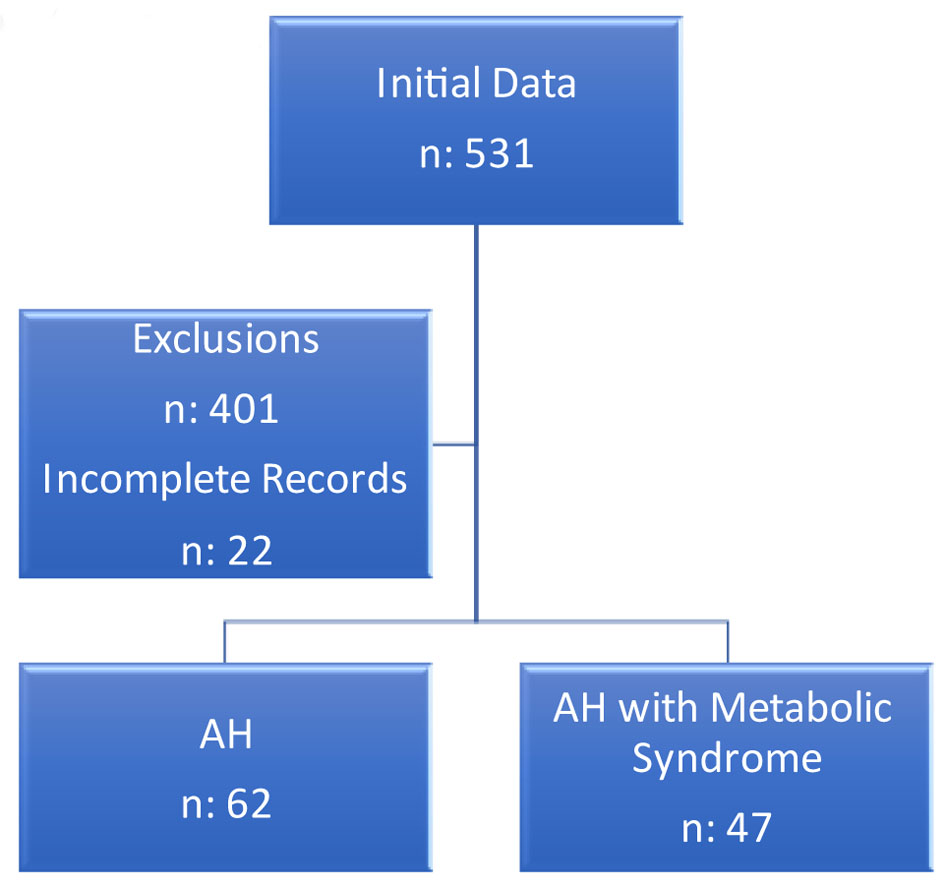 Click for large image | Figure 2. Study cohort categorization. |
Statistical analysis
Clinical features were summarized by mean ± standard deviation (SD) for continuous variables and frequency (%) for categorical variables by insulin metabolic syndrome status. Wilcoxon rank-sum tests were carried out to compare continuous variables and Fisher’s exact tests were used to compare categorical variables between the metabolic syndrome groups. Logistic regression was used to identify the factors accompanied by metabolic syndrome status. Cox regression was applied to identify the factors associated with survival and evaluate the predictability for each of the fitted models, in which C-statistic and receiver operating characteristic (ROC) curves were derived.
| Results | ▴Top |
In this study, 109 patients were included, with a mean age of 47.49 ± 12.72 years. Sixty-two patients were AH, and 47 patients were AH with metabolic syndrome. In addition, 39% of patients were women, and 72 (66%) were Caucasian. Sixty-four (58.72%) had albumin levels of less than 3.5.
Seventy-six patients (70%) had evidence of chronic liver disease on imaging. In 36 (33%) patients, infection was diagnosed based upon positive cultures, and abnormal chest X-rays. GIB was diagnosed in 12% of the cohort, though 17% underwent endoscopic evaluation. Pneumonia was the most common infection (46%) followed by urinary tract infection (32%) and others (22%). The distribution of patients based on infection status is shown in Figure 3. Two patients had been diagnosed with systolic blood pressure (SBP). Most of the patients with ascites had not had paracentesis performed during the entire course of hospitalization. Only one had been diagnosed with sepsis with positive blood culture. Cephalosporin and ciprofloxacin were the most used antibiotics, and other antibiotics included meropenem and vancomycin. Twenty-six patients had been diagnosed with acute kidney failure and five of them underwent hemodialysis. Six patients underwent transjugular intrahepatic portosystemic shunt (TIPS). Twenty-nine patients (26.61%) had a MELD score of greater than 21. Twenty-nine patients died (26.6%). Overall, 31.9% (n = 15) of patients with metabolic syndrome died while the mortality in patients without metabolic syndrome was 22.6% (n = 14).
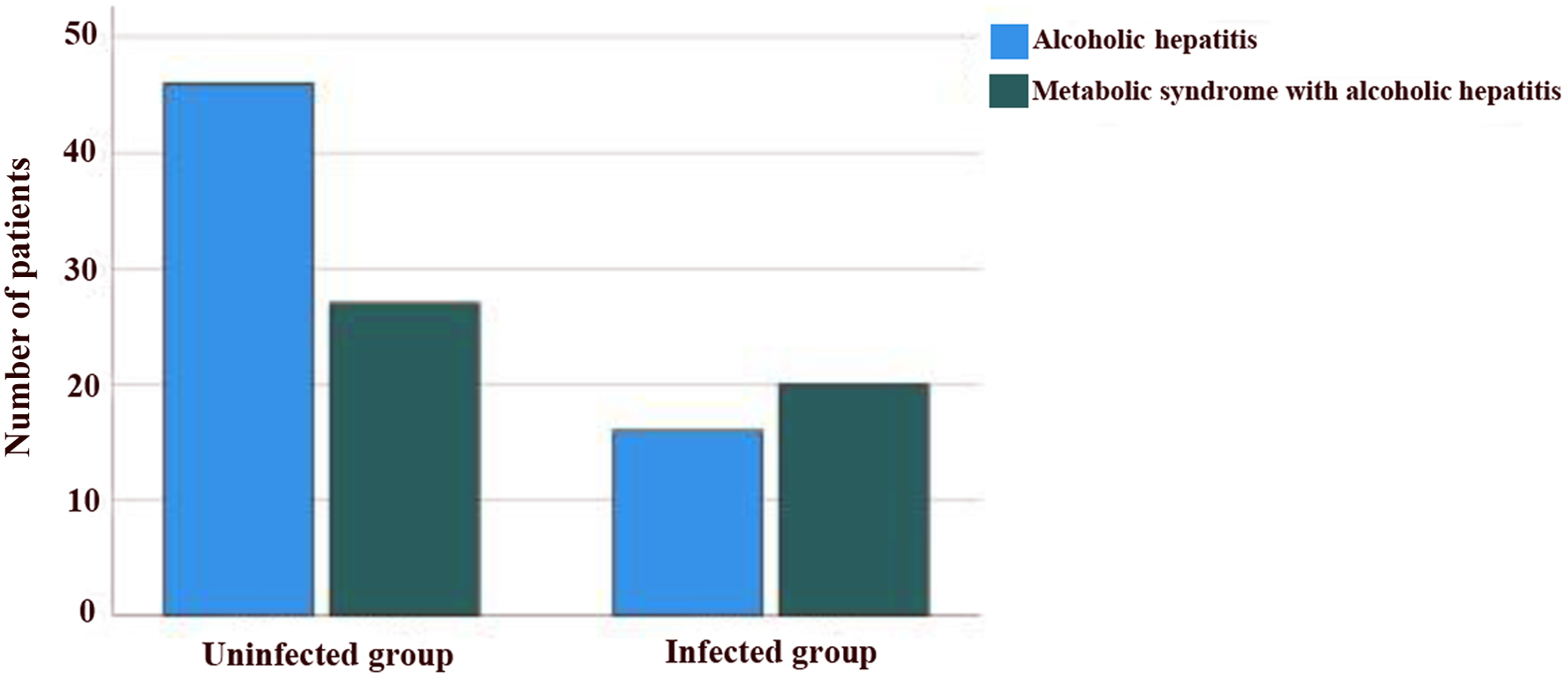 Click for large image | Figure 3. Distribution of patients based on infection status. |
Forty-one patients (37.61%) had DF ≥ 32. The results showed that the mean BMI, hemoglobin (Hb), hematocrit (HCT), and ANI were significantly different between the AH and AH with metabolic syndrome groups (P < 0.05). Also, there was a significant difference in the frequency of diabetes between the two groups (P < 0.001) (Table 1).
 Click to view | Table 1. Clinical Features Based Upon Metabolic Syndrome (t-Test, Chi-Square) |
The results of the univariate logistic regression model to identify factors associated with combined AH with metabolic syndrome showed that BMI, HCT, Hb, and ANI have a significant effect. Per unit increase in Hb, the chance of metabolic syndrome increased by 23% (odds ratio (OR) (95% confidence interval (CI)): 1.23 (1.06 - 1.43), P = 0.01). Per unit increase in BMI and HCT, metabolic syndrome increased by 26.8% respectively. However, with one unit increase of ANI, the chance of metabolic syndrome decreased by 5%. In the stepwise logistic regression model, it was shown that the BMI increased, and the chance of metabolic increased by 34% (OR (95% CI): 1.34 (1.17 - 1.54), P < 0.001) (Table 2). A bivariate correlation analysis showed that patients with AH with metabolic syndrome were more likely to have infection (43%) compared to AH (26%) with correlation coefficient of 0.176 (P = 0.03, CI: 0.018 - 1.0).
 Click to view | Table 2. Identification of Factors Associated With Combined Alcohol Hepatitis With Metabolic Syndrome (Logistic Regression, Stepwise Regression) |
Spearman’s results showed a significant positive relationship between DF score and antibiotic intake (ρ = 0.25), and there was a significant positive correlation between BMI and DF score (ρ = 0.23). In addition, there was a significant positive relationship between MELD score and infection (ρ = 0.26), between MELD score antibiotic intake (ρ = 0.27), and between age vs. MELD score (ρ = 0.20) and between BMI vs. MELD score (ρ = 0.26) (Supplementary Material 1, www.gastrores.org).
The results of the unadjusted and adjusted Cox regression model to identify factors associated with survival are shown in Table 3. The adjusted analysis showed that age, BMI, WBC, albumin levels, Na, MELD, DF score and metabolic syndrome were independently associated with mortality. Patients with a MELD greater than 18 and 21 have increased risk of death (hazard ratio (HR) (95% CI): 5.30 (2.46 - 11.40) (P < 0.001) and 5.81 (2.74 - 12.30) (P < 0.001)). Per one unit increase in blood sodium, the risk of death decreased by 8% (Table 3). The stepwise Cox regression model results showed that an increase in age, Hb, Cr, international normalized ratio (INR), and CTP increased patient mortality. However, the increase in mean corpuscular volume (MCV) significantly reduced the risk of death (Fig. 4). Mortality risks in high-risk and low-risk groups defined by MELD ≥ 21 or DF ≥ 32 with or without metabolic syndrome are shown in Figure 5a, b. Patients with metabolic syndrome in the high-risk group had the highest mortality. The effect of metabolic syndrome was significantly pronounced when risk was defined by MELD score than DF score. High-risk patients with metabolic syndrome had a higher chance of mortality than patients without metabolic syndrome. Also, in low-risk patients, those with metabolic syndrome had an almost equal chance of mortality to those without metabolic syndrome.
 Click to view | Table 3. Identification of Factors Associated With Survival (Cox Regression) |
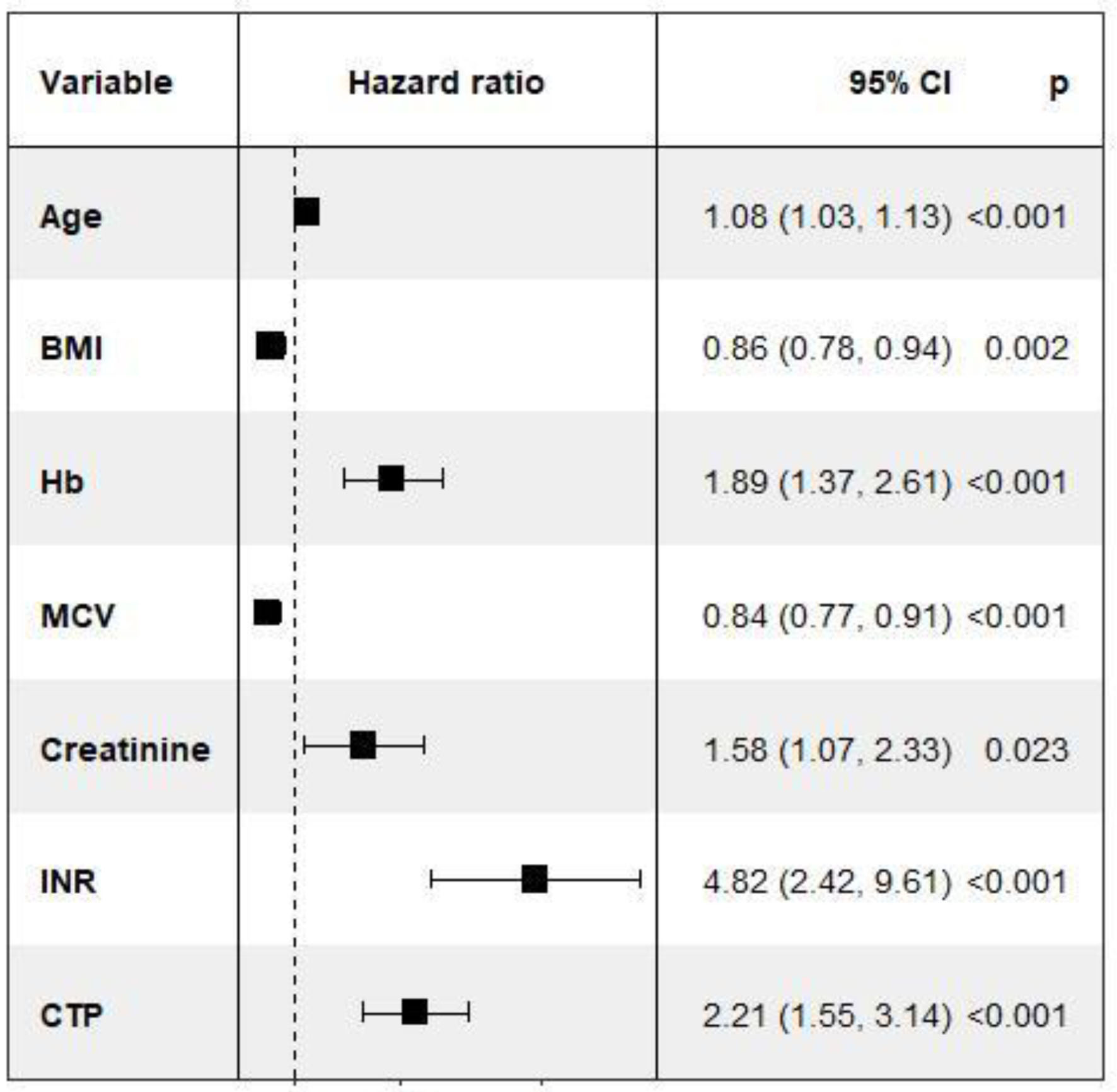 Click for large image | Figure 4. Forest plot of variables with significant P-value (< 0.05). For one unit increase of BMI, the mortality risk will decrease by 14%. BMI: body mass index. |
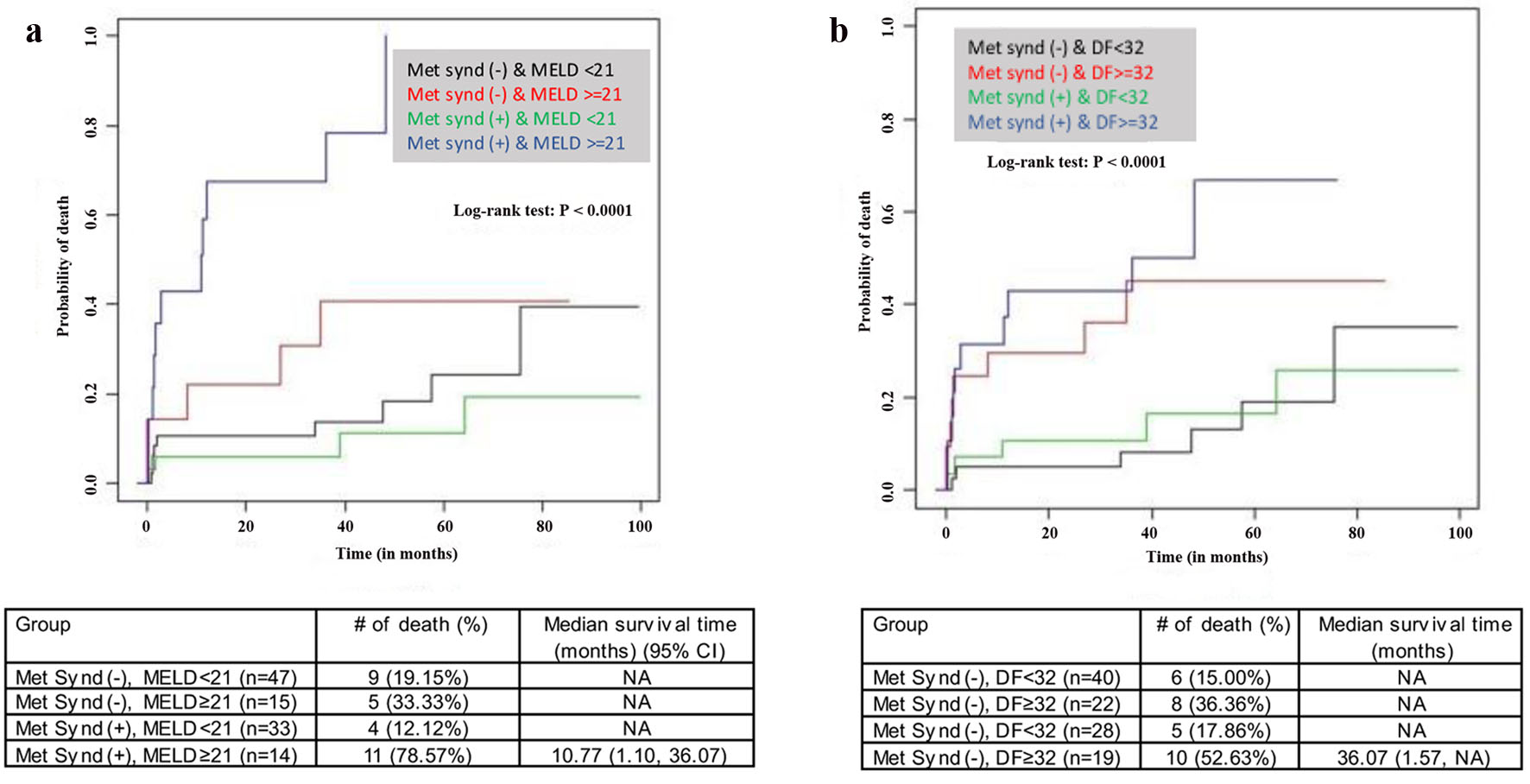 Click for large image | Figure 5. Kaplan-Meier mortality risk. (a) Stratified based upon metabolic syndrome and MELD status. (b) Stratified based upon metabolic syndrome and DF. MELD: model for end-stage liver disease; DF: discriminant function. |
Comparisons of Uno’s C-statistic between 14 of the models demonstrated that model 5, which included age, MELD ≥ 21, and albumin < 3.5, was the best model for identifying patient mortality (C-statistics = 0.82) followed by model 14 (which included DF ≥ 32, albumin < 3.5, IR and infection) with C-statistical value of 0.8 (Table 4).
 Click to view | Table 4. Comparison of Various Models to Determine Important Factors to Predict Mortality |
The ROC curves over time to visualize the model overtime prediction is shown in Supplementary Material 2 (www.gastrores.org). In the model, using age and MELD, the highest value of the area under the curve at 24 months was equal to 0.88, and the lowest value at 72 months was equal to 0.78. In the model, including age, MELD, and albumin, the highest value of the area under the curve at 24 months was equal to 0.889 and the lowest value at 72 months was equal to 0.827.
| Discussion | ▴Top |
In patients with AH, supportive care with focus on nutrition management has been standard of care for the last several decades and yet the mortality of patients with AH remains high. This demonstrates a major gap in development of new therapies and highlights the need for attention on the AH patient population. Several therapeutic approaches have been tried without conclusive results that include but are not limited to steroids, pentoxifylline, anti-TNF agents, colchicine, molecular adsorbent recirculating system (MARS) dialysis and ELAD [6-12]. Limitations in development of new treatment strategies are due to the heterogeneity of the AH patient population. Alcoholism and alcohol-related hepatoxicity may coexist in patients with other primary liver diseases such as viral hepatitis, hereditary metabolic liver disease, autoimmune liver disease, etc. Similarly, coexistence of alcoholism and severe metabolic dysfunctions defined by metabolic syndrome criteria has been reported [13-15]. When such patients with combined alcohol and metabolic syndrome present with acute deterioration, their underlying pathophysiologic mechanism of acute deterioration may not be the same as in patients with purely alcohol-related liver disease. Several factors play a role in the causation and in acute deterioration, among them are malnutrition, infections, immune dysfunction, dysbiosis, metabolic dysfunction, including insulin resistance, and toxins-induced hepatitis [22-29]. To best manage these patients, one must evaluate for such underlying pathophysiologic mechanisms and tailor the treatment based on underlying dysfunction. The currently available clinically significant diagnostic modalities are insufficient to evaluate such processes in every patient presenting with AH. Further research is needed to explore these factors.
In clinical practice, the term “alcoholic hepatitis” is used loosely, indicating a person with history of alcohol abuse and presenting with acute deterioration. We identified that three-fourths of such patients did not meet the standard diagnostic criteria set forth by ACG [3]. Such discrepancy in establishing the diagnosis and identifying the precipitants may lead to suboptimal care and undesired outcomes. Although metabolic syndrome is widespread in patients with acute AH, there are very limited data evaluating the effect of metabolic syndrome on the mortality of these patients [30, 31]. Diagnosis of metabolic syndrome in the setting of acute deterioration is challenging as acute deterioration may affect fasting blood glucose, and lipids affecting the diagnosis of metabolic syndrome, which is one of the weaknesses of the study. In our study, most of patients meeting the criteria of metabolic syndrome had known diabetes/pre-diabetes based upon A1c, BMI > 30 and h/o hypertension. Fasting blood glucose, elevated triglycerides and low HDL were incorporated in addition to the above-mentioned metabolic factors. In the high-risk cohort, we differentiated the mortality risk by presence or absence of metabolic syndrome. Our study clearly showed that mortality risk in high-risk patients (DF ≥ 32 and MELD score ≥ 21) with metabolic syndrome was the highest, whereas, in low-risk patients, the risk of mortality was comparable in patients with or without metabolic syndrome. Parker et al reported increased risk of death in obese patients with acute AH [30]. The association of metabolic syndrome and high mortality in high-risk patients was strengthened by alternative and surrogate findings. In our study, increased MCV associated with the AH group was associated with reduced mortality. Pathophysiologically, true ALD patients are malnourished, characterized by low albumin, high MCV, and a low BMI typically < 20. The ANI score is another way of identifying such patients; however, ANI does not include albumin levels. Our study also demonstrated that a lower ANI score is associated with the combined alcohol and metabolic syndrome group. This study revealed that combined ANI score and albumin can potentially identify true alcohol patients with better accuracy than ANI alone. Aligned with previous studies, the stepwise regression analysis showed that BMI is the most important factor associated with insulin resistance [32]. Our study helped differentiate both entities: the presence of renal dysfunctions and infections favors combined AH with metabolic syndrome. Moreover, we found a positive correlation between Hb and mortality. The presence of anemia favors alcoholic hepatitis, which is probably attributable to malnutrition paralleled with high MCV. Therefore, for every unit increase in Hb, the mortality risk increases by 89%. In summary, the presence of features of malnutrition favors AH and a relatively better prognosis compared with combined alcohol and metabolic syndrome.
Furthermore, the comparison between the models (Table 4) suggests that taking a more holistic approach, by looking at all predictors rather than each individual score, can improve our understanding of AH severity and efficiently detect changes in mortality risk. When we evaluated the model’s predictability for mortality prediction over time, we found that the model could predict mortality at 24 months of disease. Age, MELD ≥ 21, and albumin < 3.5 was the best model for identifying patient mortality (C-statistics = 0.82). Moreover, we found that infection occurrence and antibiotic use had a positive correlation with MELD and DF independent of other cofounders including metabolic syndrome, though prevalence of infection was high in patients with combined alcohol and metabolic syndrome. This finding was in line with Liangpunsakul et al, who showed that patients with AH who developed infections (sepsis or SBP) had a higher mortality rate than those without infections [33, 34]. Routine diagnostic criteria for acute hepatitis do not consider other precipitants of acute hepatitis, such as DILI and infections. The role of infection in the prognosis of AH patients needs further investigation.
In summary, we recommend that the MELD score, rather than the DF score, should be used as a criterion to identify disease severity. Age and serum albumin level need to be considered to define high-risk groups in AH.
Limitations
We acknowledge that this is a retrospective study with a small sample size. More data are needed to validate these findings. In addition, several parameters which were part of the metabolic syndrome definition are likely affected in the sick patients, like AH. Therefore, while we did our best to define such patients, this limitation should be considered in interpreting the data.
Conclusion
In clinical practice, diagnosis of AH is inaccurately applied. We found that insulin resistance significantly increased the mortality risk in high-risk AH. It signifies that the presence of features of metabolic syndrome modifies the behavior of AH in acute settings, warranting different therapeutic strategies. Based on our study findings, we propose that in defining AH, patients overlapping with metabolic syndrome may need to be excluded as their outcome is different with regard to risk of infections, renal dysfunctions and death.
| Supplementary Material | ▴Top |
Suppl 1. Correlation analysis between MELD and DF with other variables.
Suppl 2. ROC curves over time to visualize the predictability of the model over time for mortality prediction.
Acknowledgments
We would like to thank the University of Arizona Medical Center and the University of Arizona Liver Research Institute for providing the data for this study.
Financial Disclosure
This study is supported by the Liver Institute PLLC.
Conflict of Interest
None to declare.
Informed Consent
IRB waived the consent form requirement.
Author Contributions
Shahid Habib: principal investigator, idea, hypothesis, design, data analysis, and manuscript writing; Varun Takyar: data collection; Traci Murakami: data collection and study design; Krunal Patel: data collection; Cristian Dominguez: data collection; Omid Mehpour: manuscript writing; Chiu-Hsieh Hsu: statistical analysis.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
AH: alcoholic hepatitis; APRI: aspartate aminotransferase to platelet ratio index; ACLF: acute on chronic liver failure; AIR: alcohol insulin resistance; ANI: alcoholic liver disease/non-alcoholic fatty liver disease index; ALD: alcoholic liver disease; NAFLD: non-alcoholic fatty liver disease; MELD: model for end-stage liver disease; DF: discriminant function; MCV: mean corpuscular volume; BMI: body mass index; NASH: non-alcoholic steatohepatitis; Hb: hemoglobin; Hct: hematocrit
| References | ▴Top |
- Mendenhall CL, Moritz TE, Roselle GA, Morgan TR, Nemchausky BA, Tamburro CH, Schiff ER, et al. A study of oral nutritional support with oxandrolone in malnourished patients with alcoholic hepatitis: results of a Department of Veterans Affairs cooperative study. Hepatology. 1993;17(4):564-576.
doi pubmed - Naveau S, Giraud V, Borotto E, Aubert A, Capron F, Chaput JC. Excess weight risk factor for alcoholic liver disease. Hepatology. 1997;25(1):108-111.
doi pubmed - Singal AK, Bataller R, Ahn J, Kamath PS, Shah VH. ACG Clinical Guideline: Alcoholic Liver Disease. Am J Gastroenterol. 2018;113(2):175-194.
doi pubmed - Bozin T, Rob Z, Lucijanic M, Cmarec Buhin L, Grgurevic I. Comparison of prognostic scores for alcoholic hepatitis: a retrospective study. Croat Med J. 2021;62(1):17-24.
doi pubmed - Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119(6):1637-1648.
doi pubmed - Imperiale TF, McCullough AJ. Do corticosteroids reduce mortality from alcoholic hepatitis? A meta-analysis of the randomized trials. Ann Intern Med. 1990;113(4):299-307.
doi pubmed - Mathurin P, O'Grady J, Carithers RL, Phillips M, Louvet A, Mendenhall CL, Ramond MJ, et al. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis: meta-analysis of individual patient data. Gut. 2011;60(2):255-260.
doi pubmed - Yu CH, Xu CF, Ye H, Li L, Li YM. Early mortality of alcoholic hepatitis: a review of data from placebo-controlled clinical trials. World J Gastroenterol. 2010;16(19):2435-2439.
doi pubmed - Hosseini N, Shor J, Szabo G. Alcoholic hepatitis: A Review. Alcohol Alcohol. 2019;54(4):408-416.
doi pubmed - Hmoud BS, Patel K, Bataller R, Singal AK. Corticosteroids and occurrence of and mortality from infections in severe alcoholic hepatitis: a meta-analysis of randomized trials. Liver Int. 2016;36(5):721-728.
doi pubmed - Thompson J, Jones N, Al-Khafaji A, Malik S, Reich D, Munoz S, MacNicholas R, et al. Extracorporeal cellular therapy (ELAD) in severe alcoholic hepatitis: A multinational, prospective, controlled, randomized trial. Liver Transpl. 2018;24(3):380-393.
doi pubmed - Thursz M, Richardson P, Allison ME, et al. Steroids or pentoxifylline for alcoholic hepatitis: results of the STOPAH trial. Hepatology. 2014;60(S1):LB1.
doi - Stepanova M, Rafiq N, Younossi ZM. Components of metabolic syndrome are independent predictors of mortality in patients with chronic liver disease: a population-based study. Gut. 2010;59(10):1410-1415.
doi pubmed - Diehl AM. Obesity and alcoholic liver disease. Alcohol. 2004;34(1):81-87.
doi pubmed - Purohit V, Russo D, Coates PM. Role of fatty liver, dietary fatty acid supplements, and obesity in the progression of alcoholic liver disease: introduction and summary of the symposium. Alcohol. 2004;34(1):3-8.
doi pubmed - Lieber CS. Alcoholic fatty liver: its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol. 2004;34(1):9-19.
doi pubmed - Sakaguchi S, Takahashi S, Sasaki T, et al. Progression of alcoholic or non-alcoholic steatohepatitis; common metabolic aspects of innate immune system and oxidative stress. Review Drug Metab Pharmacokinet. 2011;26(1):30-46.
- Nagata K, Suzuki H, Sakaguchi S. Common pathogenic mechanism in development progression of liver injury caused by non-alcoholic or alcoholic steatohepatitis. J Toxicol Sci. 2007;32(5):453-468.
doi pubmed - O'Keefe EL, DiNicolantonio JJ, O'Keefe JH, Lavie CJ. Alcohol and CV Health: Jekyll and Hyde J-Curves. Prog Cardiovasc Dis. 2018;61(1):68-75.
doi pubmed - Stone NJ, Bilek S, Rosenbaum S. Recent National Cholesterol Education Program Adult Treatment Panel III update: adjustments and options. Am J Cardiol. 2005;96(4A):53E-59E.
doi pubmed - Lim JU, Lee JH, Kim JS, Hwang YI, Kim TH, Lim SY, Yoo KH, et al. Comparison of World Health Organization and Asia-Pacific body mass index classifications in COPD patients. Int J Chron Obstruct Pulmon Dis. 2017;12:2465-2475.
doi pubmed - Habib S. Letter to the Editor: Flare of autoimmune hepatitis causing acute on chronic liver failure: diagnosis and response to corticosteroid therapy. Hepatology. 2019;70(2):754-755.
doi pubmed - Habib S, Patel N, Yarlagadda S, Hsu CH, Patel S, Schader L, Walker C, et al. Safety and efficacy of antibiotics among acutely decompensated cirrhosis patients. J Gastroenterol Hepatol. 2018;33(11):1882-1888.
doi pubmed - Habib S, Yarlagadda S, Carreon TA, Schader LM, Hsu CH. Fungal infection in acutely decompensated cirrhosis patients: value of model for end-stage liver disease score. Gastroenterology Res. 2020;13(5):199-207.
doi pubmed - Kim SH, Kim BG, Kim W, Oh S, Kim HY, Jung YJ, Jeong JB, et al. Characterization of gastrointestinal hemorrhage and prediction of mortality in Asian patients with alcoholic hepatitis. J Gastroenterol Hepatol. 2016;31(4):814-821.
doi pubmed - Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144(7):1426-1437.e1421-e1429.
doi pubmed - Sarin SK, Choudhury A, Sharma MK, Maiwall R, Al Mahtab M, Rahman S, Saigal S, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hepatol Int. 2019;13(4):353-390.
doi pubmed - Arroyo V, Moreau R, Kamath PS, et al. Acute-on-chronic liver failure in cirrhosis. Nature Reviews Disease Primers. 2016;2(1):1-18.
doi pubmed - Lowe PP, Gyongyosi B, Satishchandran A, Iracheta-Vellve A, Ambade A, Cho Y, Kodys K, et al. Correction: Alcohol-related changes in the intestinal microbiome influence neutrophil infiltration, inflammation and steatosis in early alcoholic hepatitis in mice. PLoS One. 2017;12(5):e0179070.
doi pubmed - Parker R, Kim SJ, Im GY, Nahas J, Dhesi B, Vergis N, Sinha A, et al. Obesity in acute alcoholic hepatitis increases morbidity and mortality. EBioMedicine. 2019;45:511-518.
doi pubmed - Carr RM, Correnti J. Insulin resistance in clinical and experimental alcoholic liver disease. Ann N Y Acad Sci. 2015;1353(1):1-20.
doi pubmed - Sezer H. Insulin Resistance, Obesity and Lipotoxicity. In: Engin AB, Engin A (eds). Advances in Experimental Medicine and Biology, vol 960. Springer, Cham. 2017:277-304.
doi pubmed - Liangpunsakul S. Clinical characteristics and mortality of hospitalized alcoholic hepatitis patients in the United States. J Clin Gastroenterol. 2011;45(8):714-719.
doi pubmed - Sundaram V, Kaung A, Rajaram A, Lu SC, Tran TT, Nissen NN, Klein AS, et al. Obesity is independently associated with infection in hospitalised patients with end-stage liver disease. Aliment Pharmacol Ther. 2015;42(11-12):1271-1280.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


