| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Review
Volume 14, Number 1, February 2021, pages 1-12
What Do Influenza and COVID-19 Represent for Patients With Inflammatory Bowel Disease?
Sandra Maria Barbalhoa, b, c, d, Julia Novaes Matiasa, Uri Adrian Prync Flatoa, b, Joao Paulo Galletti Pilonb, Piero Bitellib, Marcos Alberto Pagani Juniorb, Antonelly Cassio Alves de Carvalhob, Jesselina Francisco dos Santos Habera, Carlos Henrique Bertoni Reisb, Ricardo de Alvares Goulartb
aDepartment of Biochemistry and Pharmacology, School of Medicine, University of Marilia (UNIMAR), Avenida Higino Muzzi Filho, 1001, Marilia, Sao Paulo, Brazil
bPostgraduate Program in Structural and Functional Interactions in Rehabilitation, UNIMAR, Marilia, SP, Brazil
cSchool of Food and Technology of Marilia (FATEC), Marilia, SP, Brazil
dCorresponding Author: Sandra Maria Barbalho, Department of Biochemistry and Pharmacology, School of Medicine, University of Marilia, Av. Higino Muzzi Filho 1001, Marilia 15525-902, SP, Brazil
Manuscript submitted December 19, 2020, accepted January 14, 2021, published online February 19, 2021
Short title: Influenza, COVID-19 and IBD
doi: https://doi.org/10.14740/gr1358
| Abstract | ▴Top |
Background: Inflammatory bowel diseases (IBD) are a group of immune and inflammatory diseases; and patients seem to be more vulnerable to influenza and coronavirus disease 2019 (COVID-19). These conditions are characterized by the augmented release of inflammatory cytokines that have been suggested as potential triggers for the acute respiratory distress syndrome, which may favor severe and even fatal outcomes. For these reasons, this review aims to evaluate what influenza and COVID-19 may represent for patients with IBD.
Methods: The search was performed in MEDLINE/PubMed, EMBASE, and Cochrane databases. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed to build the review.
Results: The conventional therapies used by IBD patients may also interfere in the outcomes of influenza and COVID-19. Immune-suppressors agents are associated with a higher risk of infections due to the inhibition of intracellular signals necessary to the host act against pathogens. On the other hand, drugs related to the suppression of the production of cytokines in IBD could bring benefits to reduce mucosal inflammation, and for preventing pneumonia. Moreover, coronaviruses can bind to the target cells through angiotensin-converting enzyme 2 (ACE-2) receptor that is expressed in epithelial cells of the lung and largely the colon and the terminal ileum suggesting that human intestinal tract could be an alternative route for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Conclusions: Once the cytokine storm observed in influenza and COVID-19 is similar to the cytokine pattern observed in IBD patients during the disease flares, the advice is that avoiding the infections is still an optimal option for IBD subjects.
Keywords: Influenza: COVID-19; Ulcerative colitis; Crohn’s disease; Inflammatory bowel disease
| Introduction | ▴Top |
Inflammatory bowel diseases (IBD) are a group of immune and inflammatory diseases, mainly represented by Crohn’s disease (CD) and ulcerative colitis (UC). The pathophysiology of these diseases remains not fully clarified; however, genetic involvement in predisposing individuals is considered. Environmental factors such as diet, smoking, alcohol consumption, and changes in the intestinal microbiome may also be involved [1-3].
There is evidence that influenza is possibly more severe in patients who have IBD and use immunomodulators so that the influenza vaccine is less effective in this group when compared to the general population, although it is strongly recommended [4, 5].
Influenza is a common respiratory illness that can be related to severe illness and death, mainly in patients with chronic inflammatory conditions, and had affected millions of people around the world [6]. Nevertheless, the new line of coronavirus (severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)) is today one of the most worrying pandemics. The method of initial transmission of the virus is possibly related to the consumption and contact with wild animals. Human-human contamination became a source of rapid and worrying dissemination and that international spreading is highlighted, as international travelers became propagators. This fact contributed to coronavirus disease 2019 (COVID-19) becomes a global issue [7, 8].
In addition to respiratory manifestations, patients infected with COVID-19 may present nausea or vomiting, or both, and diarrhea, which denote the involvement of the gastrointestinal tract by this viral infection [9].
Some risk factors were related to COVID-19, such as immunosuppressive therapy, diabetes, hypertension, older age, malnutrition, and pregnancy. Many of these treatments are related to increased risks of infections. For these reasons, patients with UC and CD should be considered a possible risk group [7, 10].
Furthermore, it is known that the virus requires the angiotensin-converting enzyme 2 (ACE-2) receptor to perform its entry into the cell. The ACE-2 receptor is found in large quantities in alveolar cells of the lungs, but the small intestine is also known to express this receptor. A study showed the presence of the virus and ACE-2 receptor in the biopsy taken in the stomach, duodenum, and rectum of a patient infected with SARS-CoV-2. Beyond that, in vitro studies have linked the increase in the expression of ACE-2 through a pro-inflammatory secretory pattern, since interferon-gamma (IFN-γ) can induce ACE-2, whose promoter region contains several cytokine responsive transcription factor binding sites [10-12].
For these reasons, this review aims to evaluate what influenza and COVID-19 may represent for patients with IBD.
| Methods | ▴Top |
Focused question
This review was performed to answer the focused question: “Are IBD patients more vulnerable to influenza or COVID-19?”
Language
Only studies in English were selected.
Databases
This review has included studies available in MEDLINE/PubMed (National Library of Medicine, National Institutes of Health), EMBASE, and Cochrane databases. The descriptors used were Influenza and Inflammatory Bowel Disease or Ulcerative Colitis or Crohn’s Disease and COVID-19 and Inflammatory Bowel Disease or Ulcerative Colitis or Crohn’s Disease. The authors of this review have followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Data extraction and selection of the studies
The inclusion criteria were studies performed in humans, including randomized clinical trials (RCTs). The exclusion criteria were reviews, studies not in English, editorials, and poster presentations.
The search for this review was performed by two independent reviewers (SMB and JNM) for the identification of studies based on the titles and abstracts of the studies. Full-text articles were evaluated to support the decision-making process if necessary. A third person (RAG) resolved disagreements between these reviewers.
The three reviewers independently performed the extraction of the characteristics of the included studies and outcomes. The PRISMA flowchart was used to expose the inclusion/exclusion retrieved records.
The Population, Intervention, Comparison, and Outcomes (PICO) were used to perform the review process.
| Results | ▴Top |
The flow diagram (Fig. 1) shows the selection of the articles, as well as the inclusion and exclusion criteria. After identifying the articles, only nine studies were selected to build this review. Altogether, 1,548 IBD patients were enrolled in the selected studies, 961 with CD, 493 with UC, 10 with indeterminate colitis, and seven with Behcet’s disease, age 6 - 75 years old. Two studies did not report the type of IBD of the patients enrolled.
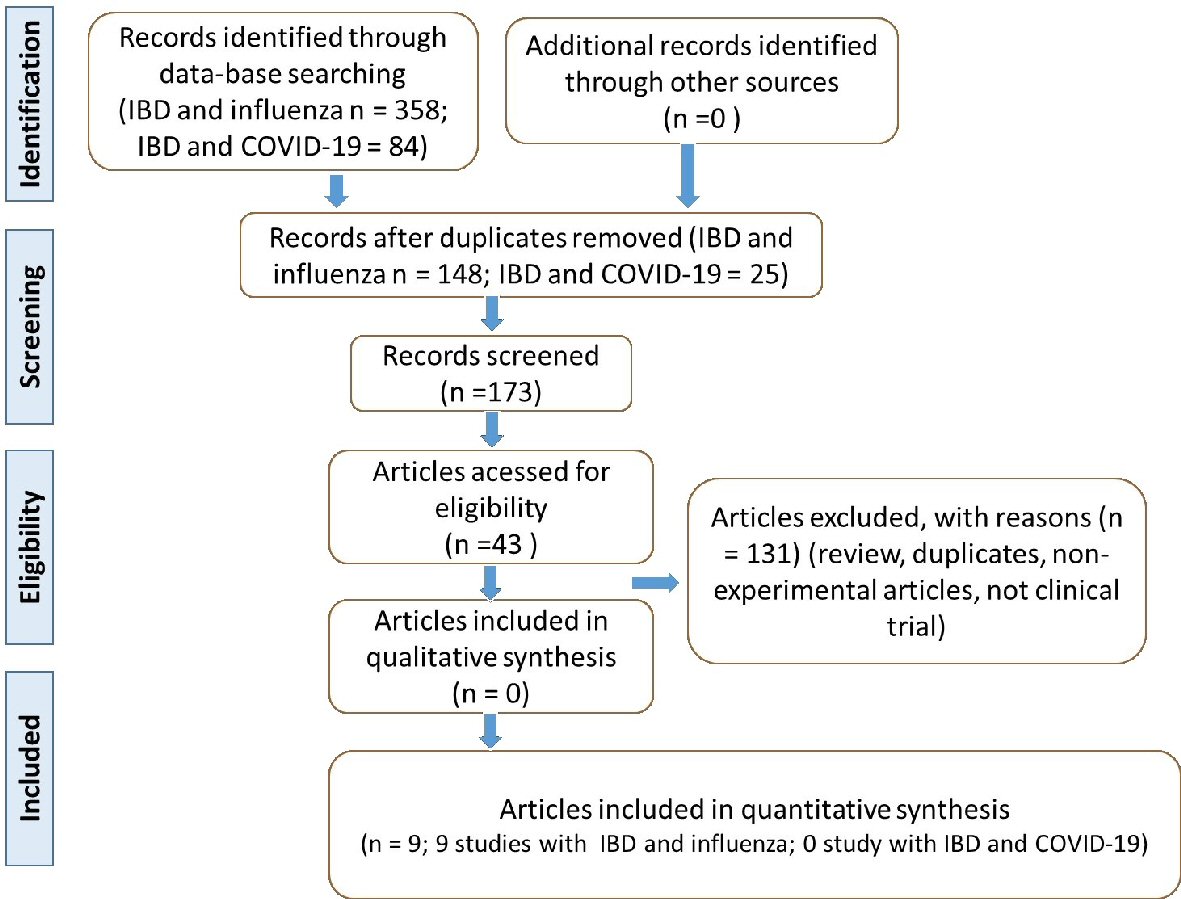 Click for large image | Figure 1. Flow diagram showing the study selection (PRISMA guidelines). IBD: inflammatory bowel disease; COVID-19: coronavirus disease 2019; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses. |
| Discussion | ▴Top |
IBD in a nutshell
The prevalence of both UC and CD are augmenting sharply over the end of the 20th century, and beginning of the 21st century, and can be considered as the most prevalent gastrointestinal disorders in industrialized countries [13, 14].
IBDs are lifelong conditions characterized by cracks in the epithelial lining (or abnormal mucus production) of some areas of the intestine; and the non-healing (defective repair) of the mucosal damage is of crucial importance. The inflammatory process related to the development of these diseases involves alterations in the permeability of the epithelial cells, microbiome patterns, and imbalanced immune response resulting in the stimulation of Tool-like receptors (TLR), dendritic cells and differentiation of CD4+T-cells into T helper cells (TH1, TH2, TH9, and TH17). The results of this process lead to the release of the pro-inflammatory (interleukin (IL)-1β), IL-4, IL-6, IL-9, IL-17, IL-23, tumor necrosis factor (TNF)-α, and interferon (IFN)-γ. This imbalanced inflammatory process disrupts the bowel homeostasis (Fig. 2). Besides the influx of macrophages and neutrophils and the over-production of inflammatory cytokines, there is also an increment in the production of proteolytic enzymes and free radicals that contribute to inflammation and may lead to ulcerations. The consequences for the patients are diarrhea, abdominal pain, bleeding, weight loss, increased risk to colon cancer, and decreased quality of life [15].
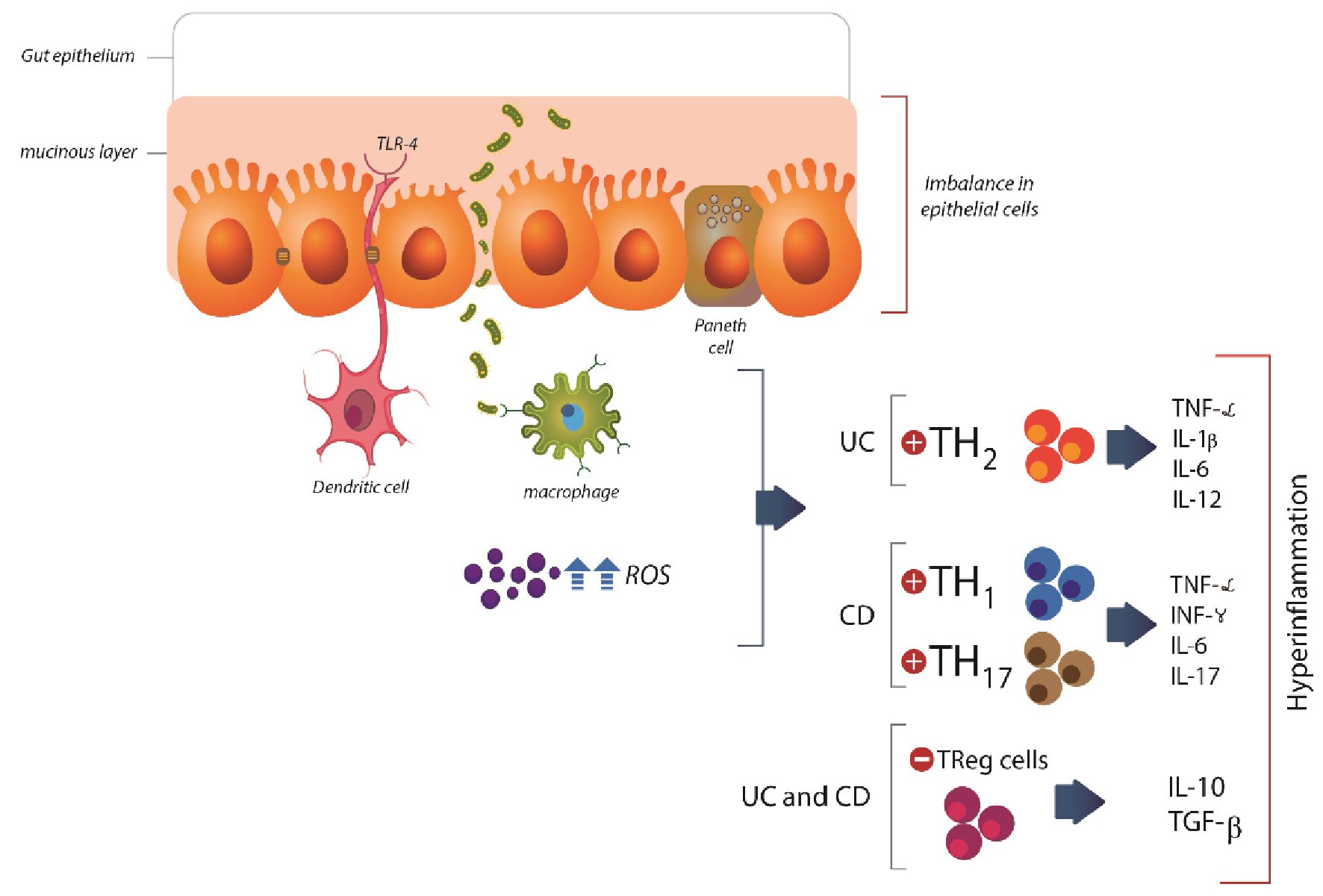 Click for large image | Figure 2. Pathophysiologic aspects of inflammatory bowel disease. The imbalance in the mucinous layer and epithelial cells leads to increased permeability to pathogens and activation of inflammatory pathways. In UC, the response is mediated by TH2, and in DC is mediated by TH1 and TH17 resulting in the synthesis of inflammatory cytokines (TNF-α, IFN-γ, IL-6, IL-17, IL-23). This scenario is also represented by a reduction in the activity of Treg cells and reduction of IL-10 and TGF-β. DC: dendritic cell; TNF-α: tumor necrosis factor-α; IFN-γ: interferon gamma; IL: interleukin; TGF-β: transforming growth factor-β; NFκβ: nuclear factor kappa β; TH: T helper cell; UC: ulcerative colitis; CD: Crohn’s disease; ROS: reactive oxygen species. |
In UC patients, the inflammatory pattern is mediated mainly by TH2 responses and limited to the colon and rectum. In patients with CD, the inflammatory process is mediated by TH1 and TH17 and involves skipped areas from mouth to anus. Phases feature both UC and CD denominated flares in which the patients present rises in the chronic inflammatory pattern, disease severity, and periods of clinical remission [16, 17].
The clinical protocol for IBD includes sulfasalazine, mesalazine, corticosteroids, antibiotics, azathioprine, methotrexate, infliximab, adalimumab, and other anti-TNF. Many of these therapies are associated with increased risk for infections, and can also interfere with the expected results of immunization against the influenza virus (Table 1 [18-26]). For these reasons, IBD patients should be considered a possible risk group regarding influenza and COVID-19 [1, 7, 10, 27, 28].
 Click to view | Table 1. Randomized Clinical Trials Showing the Effects of the Vaccine for Influenza in IBD Patients [23, 49-56] |
Influenza
Influenza viruses belong to the family orthomyxovirus. The envelope of the virus of influenza A exhibits two main surface glycoproteins named hemagglutinin A and neuraminidase. The first is responsible for membrane fusion, and the second is responsible for the release of the virions. Many health patients and IBD subjects who are infected by the influenza virus may need hospitalization that may include intensive care and sometimes interruption of the immunosuppressive drugs. Moreover, disease flares have been reported after influenza infection [29-31].
Among the subtypes of influenza A, H1N1 and H3N2 seem to mutate during the season and are related to more severe disease when compared with influenza B (B/Victoria and B/Yamagata). IBD patients are at increased risk for severe complications from influenza viruses, and the Advisory Committee on Immunization Practice and gastroenterology practice guidelines recommend annual influenza immunization. Increase in the antibody levels obtained with vaccination may reduce infection processes [18, 32]
Authors have shown that targeting influenza virus glycoprotein hemagglutinin seems to be essential protection against influenza. On the other hand, clinical trials have shown an essential role for T-cells in the achievement of protection. The concentrations of influenza-specific IFN-γ producing CD4+ and CD8+ T-cells are negatively correlated with the development or severity of the condition [33-35].
H1N1 viral infections have been reported worldwide and represent the leading cause of severe acute pneumonia, and acute respiratory distress syndrome (ARDS). For these reasons, H1N1 epidemics may represent a challenge and a severe burn to public health systems [36].
Patients with severe influenza infection present a robust increase in the levels of TNF-α, IL-6, IL-8, IFNs, and monocyte chemoattractant protein-1 (MCP-1), interferon-inducible protein-10 (IP-10), and C-C motif chemokines ligand 5 (CCL-5). This scenario of hyper-inflammation is named cytokine storm and leads patients to an increased risk of morbidity and mortality (Fig. 3) [37, 38].
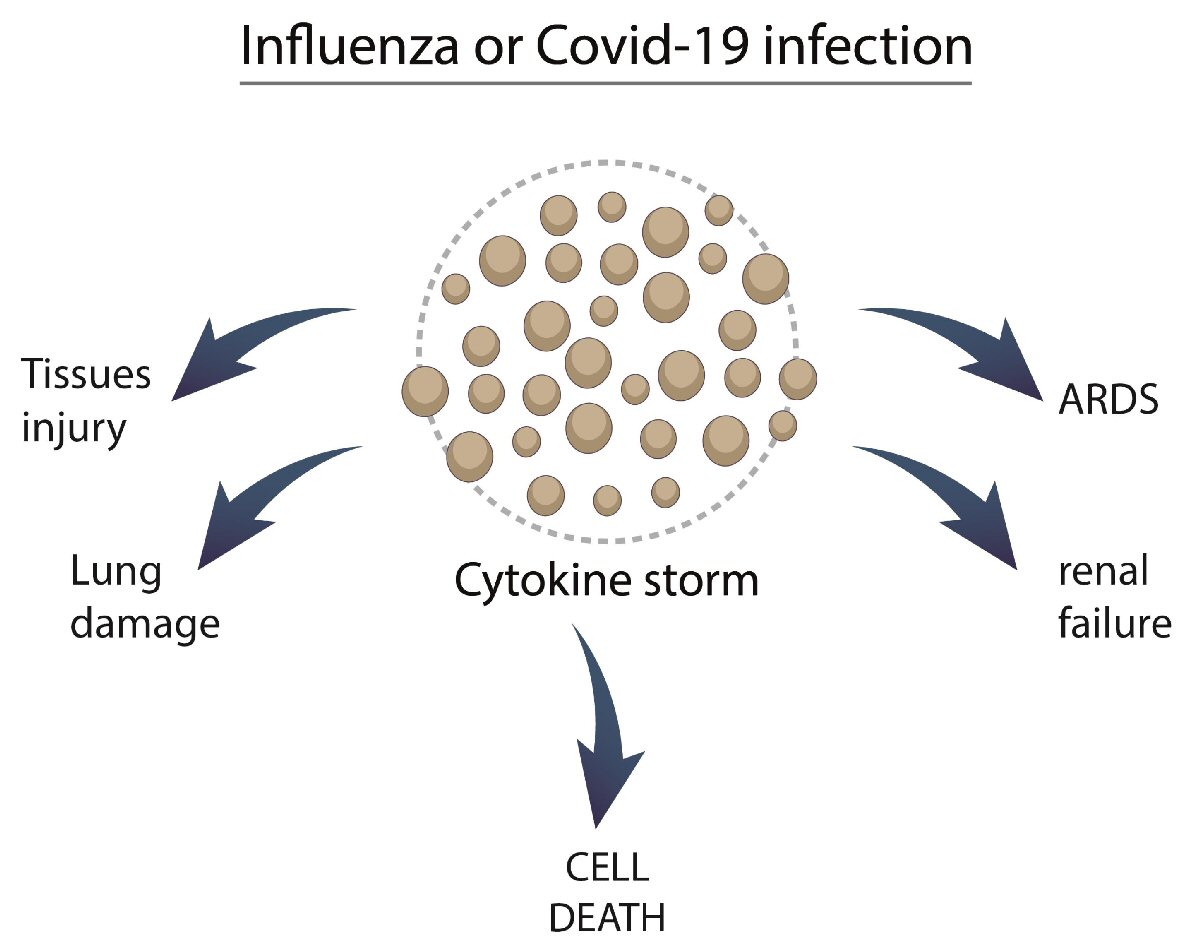 Click for large image | Figure 3. Possible mechanisms involving influenza and COVID-19 with cytokine storm. COVID-19: coronavirus disease 2019; ARDS: acute respiratory distress syndrome. |
The estimative is that 3 - 5 million cases of severe illness and about 290,000 to 650,000 deaths per year can result from the influenza infection [31, 39].
COVID-19
The SARS-CoV-2 emerged in Wuhan in December 2019 in a Chinese Province and quickly spread throughout the world, generating a pandemic. In February 2020, the International Committee on Taxonomy of Viruses named the condition as “Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)?, and the World Health Organization (WHO) designated the disease as COVID-19; and more than 20,000 cases were confirmed. On 13 January 2021, there have been 90,335,008 confirmed cases, including 1,954,336 deaths. Unfortunately the numbers are still growing, as well as the number of affected countries [40-42].
COVID-19 belongs to the Nidovirales order, coronavirus family, and is characterized by a single-stranded ribonucleic acid (RNA)-enveloped virus. Its genome is aligned with the Bat-CoV and Bat-CoV RaTG13 genome of Rhinolophus affinis. Bats, civet cats, and pangolins have been considered intermediates in transmitting the virus to humans (Fig. 4) [43, 44].
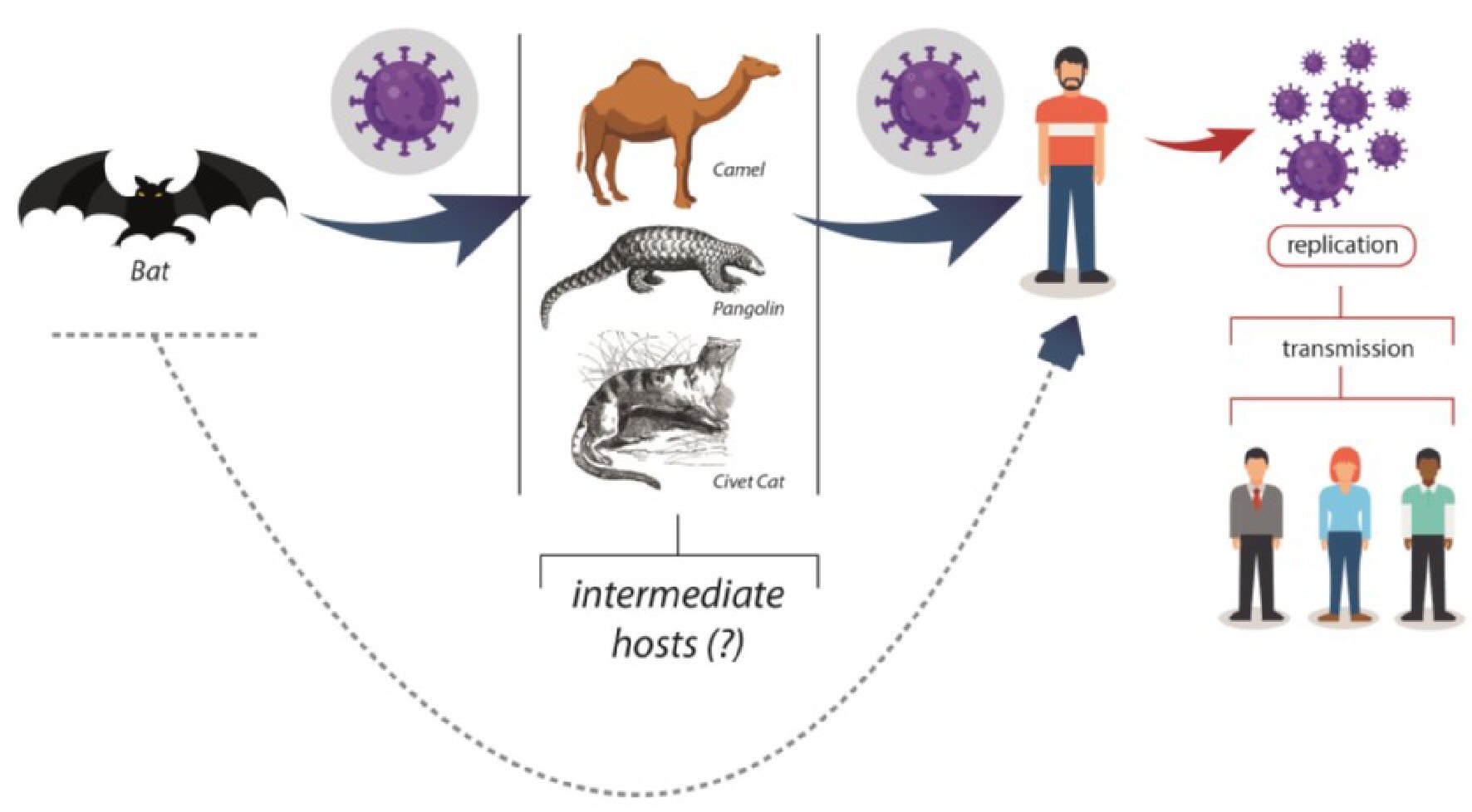 Click for large image | Figure 4. Possible origin and transmission for SARS-CoV-2 infection. SARS-CoV-2: severe acute respiratory syndrome coronavirus 2. |
COVID-19 presents four major structural proteins: the spike surface glycoprotein, small envelope protein, matrix protein, and nucleocapsid protein. The first protein is capable of binding to the host receptors through the receptor-binding domains of the ACE-2 that is present in several organs such as the respiratory system, gastrointestinal tract, thymus, lymph nodes, bone marrow, kidney, liver, and brain [45, 46].
Patients may infect other people even before the initiation of the symptoms through the saliva, respiratory droplets, urine, feces, conjunctiva, and fomites. The transmission may also occur during the clinical recovery of the disease, and the most common clinical manifestations are fever, dry cough, headache, fatigue, muscle pain, dyspnea, pneumonia, and SARS. COVID-19 pneumonia affects the chest that exhibits imaging abnormalities, even in asymptomatic patients, and a rapid evolution from focal and unilateral presentation to diffuse bilateral ground-glass pattern that progress to consolidations within 1 - 3 weeks. The symptoms also vary with the patients’ ages [47-49].
SARS-CoV or Middle East respiratory syndrome coronavirus (MERS-CoV) infected animals exhibit marked inflammatory and immune responses leading to a cytokine storm [50] (Fig. 5). The sequence of events includes a reduced release of antiviral IFNs and increased synthesis of biomarkers such as IL-1β, IL-2, IL-6, IL-8, IFN-α/β, TNF-α, IP-10, and C-C motif chemokines ligand (CCL2, CCL-3, CCL-5). Due to the genetic homology and pathologic characteristics, it is possible to predict that similar events will occur with COVID-19. Interestingly is that the anti-inflammatory IL-10 is frequently augmented in COVID-19 patients. The consequences of this scenario are apoptosis of lung epithelial and endothelial, vascular leakage, imbalanced T cell, dendritic cell, and macrophages responses that culminate in vascular alveolar edema, hypoxia, and death [41, 45, 51-53].
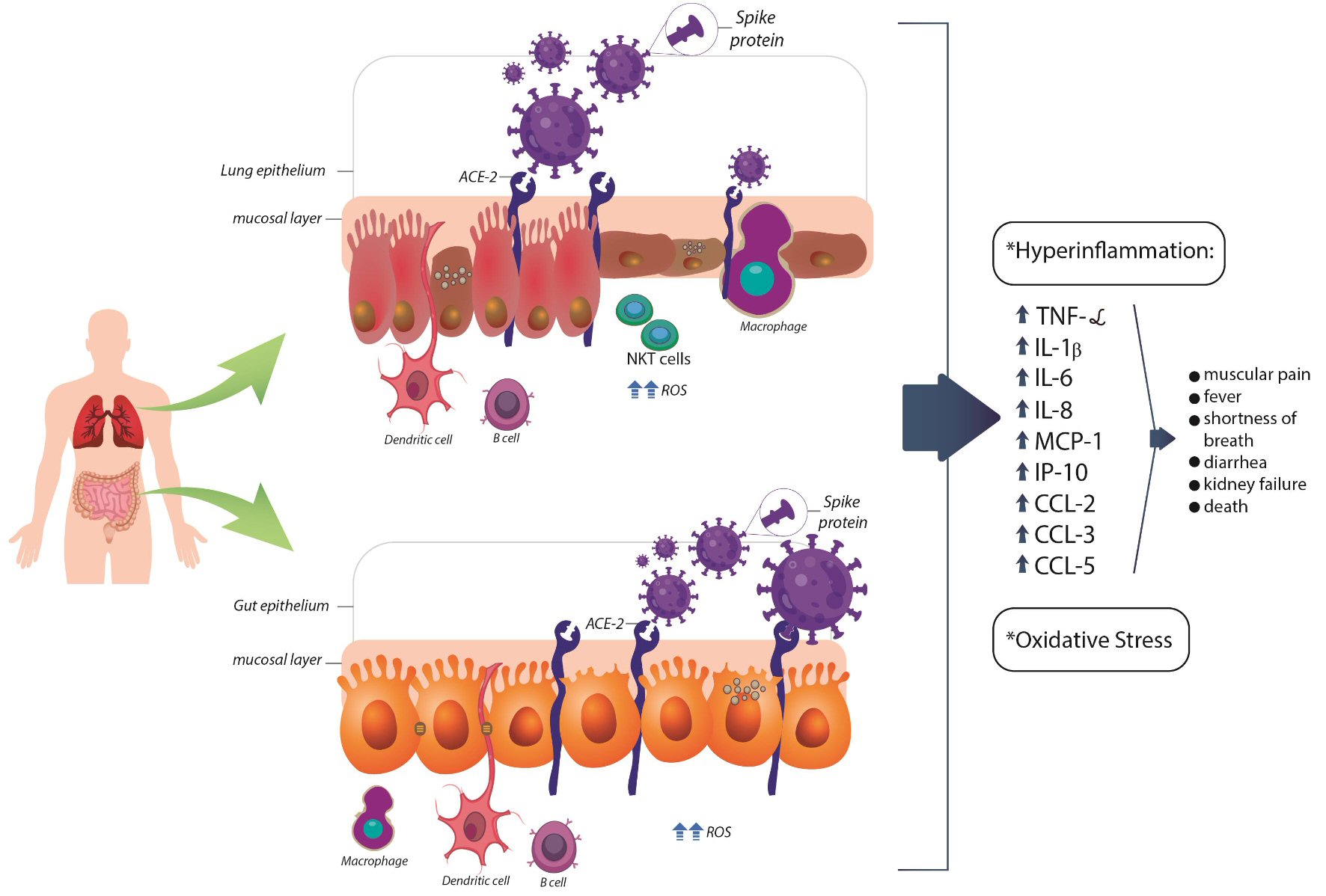 Click for large image | Figure 5. Possible pathogenesis for SARS-CoV-2 infection in lung and gut epithelium. ACE-2: angiotensin-converting enzyme 2; CCL: C-C motif chemokines ligand; IP-10: interferon-inducible protein 10; DC: dendritic cell; IFN-γ: interferon gamma; IL: interleukin; MCP-1: monocyte chemoattractant protein-1; NKT: natural killer T cell; ROS: reactive oxygen species; TNF-α: tumor necrosis factor-α. |
The release of IL-6, at high levels, has been suggested as a potential trigger for ARDS in SARS-CoV-2 infections, which may favor serious and even fatal outcomes. The levels of IFN-γ and TNF-α have also been associated with severe SARS-CoV infection [10, 54].
The cytokine storm is also associated with pronounced oxidative stress due to the synthesis of reactive oxygen species that can contribute to ARDS, which is one of the most relevant causes of deaths in the infected patients (Fig. 5) [55, 56].
Are IBD patients more vulnerable to influenza or COVID-19?
We did not find RCTs investigating IBD and COVID-19, but we can find clinical trials evaluating the influenza vaccine in these patients. Vaccine studies show that varying degrees of serologic response can be observed with vaccination (Table 1) [5, 18-26]. Higher levels of antibodies induced by vaccination can reduce the risk of illness and leads to protection against infections. One possibility to improve the immune response of the vaccine with infliximab therapy could be the use of a more concentrated vaccine [18, 57] once the administration of a booster vaccine in IBD patients has not shown to be effective [23].
Similar to influenza, COVID-19 affects the respiratory tract due to the direct viral infection or due to impairment in the immune response. An Italian study showed that hospitalizations between 2007 - 2016 for encapsulated bacterial infections were 74.5% for Streptococcus pneumoniae (S. pneumoniae), 16.3% for Neisseria meningitidis (N. meningitidis), and 9.3% for Haemophilus influenzae (H. influenzae) [58].
As pointed before, coronaviruses can bind to the target cells through ACE-2 receptor that is expressed in epithelial cells of the lung and largely the colon and the terminal ileum (amongst the highest in the body). Once the SARS-CoV-2 RNA was detected in the stool, much attention has been directed to the gastrointestinal system. More than 50% of the patients infected with COVID-19 exhibit positive tests in feces, and about one-fifth remained positive in feces even with a negative test in respiratory samples. These findings may explain the reasons why some infected patients present gastrointestinal symptoms [11].
Authors have found that diarrhea might be the first symptom before diagnosis and can occur in 49.5% of the patients, and about 22.2% of them presented this symptom before diagnosis [59].
Both key enzymes of the renin-angiotensin system (ACE and ACE-2) are expressed in high amounts in the small bowel and the colon. Some authors have found that the inflamed gut has over-expression of ACE-2 (Fig. 5). Large amounts of angiotensin I and II are found in the mucosa of recto-sigmoid biopsies obtained from patients with CD when compared to UC patients or healthy individuals. Therewithal, IBD patients have increased circulating alternative renin-angiotensin system components than patients without IBD. The treatment of these subjects with angiotensin-receptor blockers leads to reduced mucosal pro-inflammatory biomarkers than controls. Indeed, the interference in the renin-angiotensin system has been shown to play a beneficial role in some animal models of colitis [60-64], and targeting drugs could be beneficial for the human therapeutic approach.
The components of the classical and alternative axes of the renin-angiotensin system were found in the intestinal wall of IBD patients and controls, suggesting that all the components can be synthesized within the gastrointestinal tract, and probably has crucial actions in the maintenance of homeostasis or can be perturbed in intestinal inflammation [60].
For the establishment of SARS-CoV-2 infection, there must be a connection with the ACE-2 and the fusion of the capsule of the virus with the host cell membranes (Fig. 5). This process occurs through the spike surface glycoprotein, in which activation is mediated by a proteolytic cleavage induced by host cell proteases whose activity is up-regulated in IBD patients [65]. It is also noteworthy to say that there are two isoforms of ACE-2. One is anchored to the membrane (and may serve as SARS-CoV-2 receptor), and the other is circulating. The soluble form seems to compete with SARS-CoV, inhibiting the binding of the viral particle to the surface-bound in the other isoform. Noticeable is that the levels of the soluble protein are up-regulated in the peripheral blood of patients with IBD, suggesting a possible contribution to reducing the virus infection [66-68]. Indeed, with these observations it is possible to suggest that human intestinal tract could be an alternative route for SARS-CoV-2 [69, 70].
The conventional therapies used by IBD patients may also interfere in the COVID-19 infection. Immune-suppressors agents are associated with a higher risk of infections due to the inhibition of intracellular signals necessary to the host act against pathogens. Still, it is necessary to remember that the drugs related to the suppression of the production of cytokines in IBD could bring benefits both to reduce mucosal inflammation and for preventing pneumonia caused by COVID-19. Increased risk of pneumonia has already been reported in patients with IBD compared to healthy individuals, and this relationship is even more evident in patients using medications such as corticosteroids [49, 71-73].
Moreover, TNF and IL-6 targets can increase the risk for bacterial infections but show fewer effects on viral infections such as influenza. The drugs that target pro-inflammatory cytokines such as infliximab, adalimumab, ustekinumab, besides being immune suppressors and could be considered harmful in COVID-19 infection, are also associated to the capacity or neutralizing individual mediators of the inflammation process and may mitigate the hyper-inflammatory state observed in the severity of COVID-19 pathophysiology. The Janus kinase (JAK1 and 3) inhibitors used for the therapeutic approach of CD patients can interfere in the function of cytokines involved in antiviral responses such as type I INF, IL-2, IL-15, and IL-21. These inhibitors could, at least in part, inhibit the clearance of SARS-CoV-2. On the other hand, the inhibition of JAK2 seems to block the viral entrance of SARS-CoV-2 [72].
Authors also have shown that COVID-19 patients with gastrointestinal symptoms present lengthy illness duration, and it seems that the average age of positive test in the stool is 49 years, suggesting that aging is related to the ease of virus invasion [74, 75].
The above discussion may suggest that the inflamed gut of IBD patients could be an optimal door to the entrance of the virus in humans. Nevertheless, there is still much lack of evidence that the over-expression of ACE-2 in the colon and ileum could influence the entry and replication of the virus in the intestinal cells and facilitates its transmission in another extra-respiratory route [66].
Bezzio et al suggested that the presence of active IBD, older age patients with comorbidities have been associated with a higher risk of development of COVID-19 pneumonia and death in IBD patients. Moreover, concomitant treatment with immunosuppressants and biologics did not associate with worse COVID-19 prognosis in these patients [76].
According to Nakase et al, there is still no evidence that IBD itself can augment the risk of SARS-CoV-2 infection; and, for these reasons, there is no need for doctors to suddenly discontinue immunomodulatory or biologic treatment in quiescent IBD subjects. Besides that, there is a need for careful observation of older age patients (> 60 years old) and those subjects receiving corticosteroid therapy during the COVID-19 pandemic [77].
Evidence shows that COVID-19 can exacerbate symptoms of IBD, and it is important to distinguish between an IBD exacerbation and symptoms caused by COVID-19. Patients with active severe IBD and with COVID-19 can go through progressive pneumonia, ARDS and multi-organ failure due to the cytokine storm syndrome associated with hyper-inflammation [78, 79].
Regardless, once the cytokine storm observed in COVID-19 patients is similar to the cytokine pattern observed in the acute period of the inflammatory process of IBD patients (during the disease flares), the advice is that avoiding the infection is still an optimal option for IBD subjects.
Acknowledgments
We acknowledge Renato Vono for drawing the figures.
Financial Disclosure
The authors declare no funding support.
Conflict of Interest
The authors declare no conflict of interest.
Author Contributions
Conceptualization and design: SMB, JNM, UAPF, and RAG; methodology: SMB, JNM and RAG; writing (original draft preparation): SMB, JPGP, JFSH, CHR, PB; writing (review and editing): SMB, MAPJ, and ACAC. All authors have read and agreed to the published version of the manuscript.
Data Availability
Any inquiries about supporting data availability of this review should be directed to Doctor Sandra M. Barbalho, smbarbalho@gmail.com.
| References | ▴Top |
- Huppe A, Langbrandtner J, Lill C, Raspe H. The effectiveness of actively induced medical rehabilitation in chronic inflammatory bowel disease. Dtsch Arztebl Int. 2020;117(6):89-96.
doi pubmed - Charoenngam N, Shirvani A, Kalajian TA, Song A, Holick MF. The effect of various doses of oral vitamin D3 supplementation on gut microbiota in healthy adults: a randomized, double-blinded, dose-response study. Anticancer Res. 2020;40(1):551-556.
doi pubmed - Marton LT, Goulart RA, Carvalho ACA, Barbalho SM. Omega fatty acids and inflammatory bowel diseases: an overview. Int J Mol Sci. 2019;20(19):4851.
doi pubmed - Arihiro S, Nakashima A, Matsuoka M, Suto S, Uchiyama K, Kato T, Mitobe J, et al. Randomized trial of vitamin D supplementation to prevent seasonal influenza and upper respiratory infection in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2019;25(6):1088-1095.
doi pubmed - Boltin D, Gingold-Belfer R, Kimchi NA, Ben-Bassat O, Niv Y, Birkenfeld S. Utilization of influenza immunization in adults with Crohn's disease-a longitudinal, population-based study. Inflamm Bowel Dis. 2014;20(2):240-245.
doi pubmed - Jester BJ, Uyeki TM, Jernigan DB. Fifty years of influenza A(H3N2) following the pandemic of 1968. Am J Public Health. 2020;110(5):669-676.
doi pubmed - Magro F, Abreu C, Rahier JF. The daily impact of COVID-19 in gastroenterology. United European Gastroenterol J. 2020;8(5):520-527.
doi pubmed - Di Gennaro F, Pizzol D, Marotta C, Antunes M, Racalbuto V, Veronese N, Smith L. Coronavirus Diseases (COVID-19) Current Status and Future Perspectives: A Narrative Review. Int J Environ Res Public Health. 2020;17(8):2690.
doi pubmed - Mao R, Liang J, Shen J, Ghosh S, Zhu LR, Yang H, Wu KC, et al. Implications of COVID-19 for patients with pre-existing digestive diseases. Lancet Gastroenterol Hepatol. 2020;5(5):425-427.
doi - Rubin DT, Abreu MT, Rai V, Siegel CA, International Organization for the Study of Inflammatory Bowel D. Management of patients with Crohn's disease and ulcerative colitis during the coronavirus disease-2019 pandemic: results of an international meeting. Gastroenterology. 2020;159(1):6-13 e16.
- Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology. 2020;158(6):1831-1833 e1833.
doi pubmed - Wang PH. Increasing host cellular receptor-angiotensin-converting enzyme 2 (ACE2) expression by coronavirus may facilitate 2019-nCoV infection. bioRxiv. 2020.
doi - Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, Panaccione R, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2018;390(10114):2769-2778.
doi - Guan Q. A comprehensive review and update on the pathogenesis of inflammatory bowel disease. J Immunol Res. 2019;2019:7247238.
doi pubmed - Barbalho SM, Bosso H, Salzedas-Pescinini LM, de Alvares Goulart R. Green tea: A possibility in the therapeutic approach of inflammatory bowel diseases?: Green tea and inflammatory bowel diseases. Complement Ther Med. 2019;43:148-153.
doi pubmed - Huang T, Okauchi T, Hu D, Shigeta M, Wu Y, Wada Y, Hayashinaka E, et al. Pain matrix shift in the rat brain following persistent colonic inflammation revealed by voxel-based statistical analysis. Mol Pain. 2019;15:1744806919891327.
doi pubmed - Hanzel J, D'Haens GR. Anti-interleukin-23 agents for the treatment of ulcerative colitis. Expert Opin Biol Ther. 2020;20(4):399-406.
doi pubmed - Caldera F, Hillman L, Saha S, Wald A, Grimes I, Zhang Y, Sharpe AR, et al. Immunogenicity of high dose influenza vaccine for patients with inflammatory bowel disease on anti-TNF monotherapy: a randomized clinical trial. Inflamm Bowel Dis. 2020;26(4):593-602.
doi pubmed - Romanowska M, Banaszkiewicz A, Nowak I, Radzikowski A, Brydak LB. Immunization against influenza during the 2005/2006 epidemic season and the humoral response in children with diagnosed inflammatory bowel disease (IBD). Med Sci Monit. 2010;16(9):CR433-439.
- Rahier JF, Papay P, Salleron J, Sebastian S, Marzo M, Peyrin-Biroulet L, Garcia-Sanchez V, et al. H1N1 vaccines in a large observational cohort of patients with inflammatory bowel disease treated with immunomodulators and biological therapy. Gut. 2011;60(4):456-462.
doi pubmed - Andrisani G, Frasca D, Romero M, Armuzzi A, Felice C, Marzo M, Pugliese D, et al. Immune response to influenza A/H1N1 vaccine in inflammatory bowel disease patients treated with anti TNF-alpha agents: effects of combined therapy with immunosuppressants. J Crohns Colitis. 2013;7(4):301-307.
doi pubmed - Balint A, Farkas K, Eva PK, Terhes G, Urban E, Szucs M, Nyari T, et al. Antibody and cell-mediated immune response to whole virion and split virion influenza vaccine in patients with inflammatory bowel disease on maintenance immunosuppressive and biological therapy. Scand J Gastroenterol. 2015;50(2):174-181.
doi pubmed - Matsumoto H, Ohfuji S, Watanabe K, Yamagami H, Fukushima W, Maeda K, Kamata N, et al. Booster influenza vaccination does not improve immune response in adult inflammatory bowel disease patients treated with immunosuppressives: a randomized controlled trial. J Gastroenterol. 2015;50(8):876-886.
doi pubmed - Launay O, Abitbol V, Krivine A, Slama LB, Bourreille A, Dupas JL, Hebuterne X, et al. Immunogenicity and safety of influenza vaccine in inflammatory bowel disease patients treated or not with immunomodulators and/or biologics: a two-year prospective study. J Crohns Colitis. 2015;9(12):1096-1107.
doi pubmed - deBruyn J, Fonseca K, Ghosh S, Panaccione R, Gasia MF, Ueno A, Kaplan GG, et al. Immunogenicity of influenza vaccine for patients with inflammatory bowel disease on maintenance infliximab therapy: a randomized trial. Inflamm Bowel Dis. 2016;22(3):638-647.
doi pubmed - Shirai S, Hara M, Sakata Y, Tsuruoka N, Yamamoto K, Shimoda R, Gomi Y, et al. Immunogenicity of quadrivalent influenza vaccine for patients with inflammatory bowel disease undergoing immunosuppressive therapy. Inflamm Bowel Dis. 2018;24(5):1082-1091.
doi pubmed - Rapport F, Clement C, Seagrove AC, Alrubaiy L, Hutchings HA, Williams JG. Patient views about the impact of ulcerative colitis and its management with drug treatment and surgery: a nested qualitative study within the CONSTRUCT trial. BMC Gastroenterol. 2019;19(1):166.
doi pubmed - Cunha Neto F, Marton LT, de Marqui SV, Lima TA, Barbalho SM. Curcuminoids from Curcuma Longa: New adjuvants for the treatment of crohn's disease and ulcerative colitis? Crit Rev Food Sci Nutr. 2019;59(13):2136-2143.
doi pubmed - Rahier JF, Papay P, Salleron J, Sebastian S, Ellul P, Teich N, Fiorino G, et al. Influenza A (H1N1)v infection in patients with inflammatory bowel disease: a case series. Aliment Pharmacol Ther. 2011;33(4):499-500.
doi pubmed - Aghaali M, Kavousi A, Shahsavani A, Hashemi Nazari SS. Performance of Bayesian outbreak detection algorithm in the syndromic surveillance of influenza-like illness in small region. Transbound Emerg Dis. 2020;67(5):2183-2189.
doi pubmed - Cantan B, Luyt CE, Martin-Loeches I. Influenza infections and emergent viral infections in intensive care unit. Semin Respir Crit Care Med. 2019;40(4):488-497.
doi pubmed - Grohskopf LA, Alyanak E, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices - United States, 2019-20 Influenza Season. MMWR Recomm Rep. 2019;68(3):1-21.
doi pubmed - Skibinski DAG, Jones LA, Zhu YO, Xue LW, Au B, Lee B, Naim ANM, et al. Induction of human T-cell and cytokine responses following vaccination with a novel influenza vaccine. Sci Rep. 2018;8(1):18007.
doi pubmed - Ho HJ, Tan YR, Cook AR, Koh G, Tham TY, Anwar E, Hui Chiang GS, et al. Increasing influenza and pneumococcal vaccination uptake in seniors using point-of-care informational interventions in primary care in Singapore: a pragmatic, cluster-randomized crossover trial. Am J Public Health. 2019;109(12):1776-1783.
doi pubmed - Tinsley A, Navabi S, Williams ED, Liu G, Kong L, Coates MD, Clarke K. Increased risk of influenza and influenza-related complications among 140,480 patients with inflammatory bowel disease. Inflamm Bowel Dis. 2019;25(2):369-376.
doi pubmed - Zhao C, Chen J, Cheng L, Xu K, Yang Y, Su X. Deficiency of HIF-1alpha enhances influenza A virus replication by promoting autophagy in alveolar type II epithelial cells. Emerg Microbes Infect. 2020;9(1):691-706.
doi pubmed - Chen S, Liu G, Chen J, Hu A, Zhang L, Sun W, Tang W, et al. Ponatinib protects mice from lethal influenza infection by suppressing cytokine storm. Front Immunol. 2019;10:1393.
doi pubmed - Herold S, Becker C, Ridge KM, Budinger GR. Influenza virus-induced lung injury: pathogenesis and implications for treatment. Eur Respir J. 2015;45(5):1463-1478.
doi pubmed - Jagadesh A, Salam AA, Mudgal PP, Arunkumar G. Influenza virus neuraminidase (NA): a target for antivirals and vaccines. Arch Virol. 2016;161(8):2087-2094.
doi pubmed - Huang F, Zhang C, Liu Q, Zhao Y, Zhang Y, Qin Y, Li X, et al. Identification of amitriptyline HCl, flavin adenine dinucleotide, azacitidine and calcitriol as repurposing drugs for influenza A H5N1 virus-induced lung injury. PLoS Pathog. 2020;16(3):e1008341.
doi pubmed - Rizzo P, Vieceli Dalla Sega F, Fortini F, Marracino L, Rapezzi C, Ferrari R. COVID-19 in the heart and the lungs: could we "Notch" the inflammatory storm? Basic Res Cardiol. 2020;115(3):31.
doi pubmed - World Health Organization (WHO). Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019?gclid=CjwKCAiAxp-ABhALEiwAXm6IyZh9QsQcJmLhUumRqQaQ8mDhvi5xYqnlzoir11ykh5wNlkKZ9G_8ABoCLyAQAvD_BwE.
- Paybast S, Emami A, Koosha M, Baghalha F. Novel coronavirus disease (COVID-19) and central nervous system complications: what neurologist need to know. Acta Neurol Taiwan. 2020;29(1):24-31.
- Kakodkar P, Kaka N, Baig MN. A comprehensive literature review on the clinical presentation, and management of the pandemic coronavirus disease 2019 (COVID-19). Cureus. 2020;12(4):e7560.
doi - Tian S, Xiong Y, Liu H, Niu L, Guo J, Liao M, Xiao SY. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol. 2020;33(6):1007-1014.
doi pubmed - Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, Zhang Q, et al. Crystal structure of the 2019-nCoV spike receptor-binding domain bound with the ACE2 receptor. bioRxiv. 2020.
doi - Respiratory Care Committee of Chinese Thoracic Society. [Expert consensus on preventing nosocomial transmission during respiratory care for critically ill patients infected by 2019 novel coronavirus pneumonia]. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43(4):288-296.
- Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R. COVID-19 infection: Origin, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91-98.
doi pubmed - Fiorino G, Allocca M, Furfaro F, Gilardi D, Zilli A, Radice S, Spinelli A, et al. Inflammatory bowel disease care in the COVID-19 pandemic era: the humanitas, Milan, experience. J Crohns Colitis. 2020;14(9):1330-1333.
doi pubmed - Schett G, Sticherling M, Neurath MF. COVID-19: risk for cytokine targeting in chronic inflammatory diseases? Nat Rev Immunol. 2020;20(5):271-272.
doi pubmed - Zhang R, Wang X, Ni L, Di X, Ma B, Niu S, Liu C, et al. COVID-19: Melatonin as a potential adjuvant treatment. Life Sci. 2020;250:117583.
doi pubmed - Ye Q, Wang B, Mao J. The pathogenesis and treatment of the 'Cytokine Storm' in COVID-19. J Infect. 2020;80(6):607-613.
doi pubmed - Channappanavar R, Fehr AR, Vijay R, Mack M, Zhao J, Meyerholz DK, Perlman S. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe. 2016;19(2):181-193.
doi pubmed - Li CK, Wu H, Yan H, Ma S, Wang L, Zhang M, Tang X, et al. T cell responses to whole SARS coronavirus in humans. J Immunol. 2008;181(8):5490-5500.
doi pubmed - Hedrich CM. COVID-19 - Considerations for the paediatric rheumatologist. Clin Immunol. 2020;214:108420.
doi pubmed - Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, Wang T, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620-2629.
doi pubmed - Syal G, Melmed GY. Is it time for high dose influenza vaccination in inflammatory bowel diseases? Inflamm Bowel Dis. 2020;26(4):603-605.
doi pubmed - Lenti MV, Mengoli C, Vernero M, Aronico N, Conti L, Borrelli de Andreis F, Cococcia S, et al. Preventing infections by encapsulated bacteria through vaccine prophylaxis in inflammatory bowel disease. Front Immunol. 2020;11:485.
doi pubmed - Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069.
doi pubmed - Garg M, Royce SG, Tikellis C, Shallue C, Batu D, Velkoska E, Burrell LM, et al. Imbalance of the renin-angiotensin system may contribute to inflammation and fibrosis in IBD: a novel therapeutic target? Gut. 2020;69(5):841-851.
doi pubmed - Garg M, Angus PW, Burrell LM, Herath C, Gibson PR, Lubel JS. Review article: the pathophysiological roles of the renin-angiotensin system in the gastrointestinal tract. Aliment Pharmacol Ther. 2012;35(4):414-428.
doi pubmed - Katada K, Yoshida N, Suzuki T, Okuda T, Mizushima K, Takagi T, Ichikawa H, et al. Dextran sulfate sodium-induced acute colonic inflammation in angiotensin II type 1a receptor deficient mice. Inflamm Res. 2008;57(2):84-91.
doi pubmed - Spencer AU, Yang H, Haxhija EQ, Wildhaber BE, Greenson JK, Teitelbaum DH. Reduced severity of a mouse colitis model with angiotensin converting enzyme inhibition. Dig Dis Sci. 2007;52(4):1060-1070.
doi pubmed - Khajah MA, Fateel MM, Ananthalakshmi KV, Luqmani YA. Anti-inflammatory action of angiotensin 1-7 in experimental colitis may be mediated through modulation of serum cytokines/chemokines and immune cell functions. Dev Comp Immunol. 2017;74:200-208.
doi pubmed - Jablaoui A, Kriaa A, Mkaouar H, Akermi N, Soussou S, Wysocka M, Woloszyn D, et al. Fecal serine protease profiling in inflammatory bowel diseases. Front Cell Infect Microbiol. 2020;10:21.
doi pubmed - Monteleone G, Ardizzone S. Are patients with inflammatory bowel disease at increased risk for COVID-19 Infection? J Crohns Colitis. 2020;14(9):1334-1336.
doi pubmed - Batlle D, Wysocki J, Satchell K. Soluble angiotensin-converting enzyme 2: a potential approach for coronavirus infection therapy? Clin Sci (Lond). 2020;134(5):543-545.
doi pubmed - Garg M, Burrell LM, Velkoska E, Griggs K, Angus PW, Gibson PR, Lubel JS. Upregulation of circulating components of the alternative renin-angiotensin system in inflammatory bowel disease: A pilot study. J Renin Angiotensin Aldosterone Syst. 2015;16(3):559-569.
doi pubmed - Monteleone G, Franze E, Laudisi F. Expression of receptors for SARS-CoV-2 in the gut of patients with inflammatory bowel disease. Gut Liver. 2020;14(4):530-531.
doi pubmed - Wong SH, Lui RN, Sung JJ. COVID-19 and the digestive system. J Gastroenterol Hepatol. 2020;35(5):744-748.
doi pubmed - Monteleone G, Pallone F, MacDonald TT. Emerging immunological targets in inflammatory bowel disease. Curr Opin Pharmacol. 2011;11(6):640-645.
doi pubmed - Russell B, Moss C, George G, Santaolalla A, Cope A, Papa S, Van Hemelrijck M. Associations between immune-suppressive and stimulating drugs and novel COVID-19-a systematic review of current evidence. Ecancermedicalscience. 2020;14:1022.
doi pubmed - Di Giambenedetto S, Ciccullo A, Borghetti A, Gambassi G, Landi F, Visconti E, Zileri Dal Verme L, et al. Off-label use of tocilizumab in patients with SARS-CoV-2 infection. J Med Virol. 2020;92(10):1787-1788.
doi pubmed - Nobel YR, Phipps M, Zucker J, Lebwohl B, Wang TC, Sobieszczyk ME, Freedberg DE. Gastrointestinal symptoms and coronavirus disease 2019: a case-control study from the United States. Gastroenterology. 2020;159(1):373-375 e372.
doi pubmed - Uno Y. Why Does SARS-CoV-2 Invade the Gastrointestinal Epithelium? Gastroenterology. 2020;159(4):1622-1623.
doi pubmed - Bezzio C, Saibeni S, Variola A, Allocca M, Massari A, Gerardi V, Casini V, et al. Outcomes of COVID-19 in 79 patients with IBD in Italy: an IG-IBD study. Gut. 2020;69(7):1213-1217.
doi pubmed - Nakase H, Matsumoto T, Matsuura M, Iijima H, Matsuoka K, Ohmiya N, Ishihara S, et al. Expert opinions on the current therapeutic management of inflammatory bowel disease during the COVID-19 pandemic: Japan IBD COVID-19 taskforce, intractable diseases, the health and labor sciences research. Digestion. 2020:1-9.
doi pubmed - Neurath MF. COVID-19 and immunomodulation in IBD. Gut. 2020;69(7):1335-1342.
doi pubmed - Al-Ani AH, Prentice RE, Rentsch CA, Johnson D, Ardalan Z, Heerasing N, Garg M, et al. Review article: prevention, diagnosis and management of COVID-19 in the IBD patient. Aliment Pharmacol Ther. 2020;52(1):54-72.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


