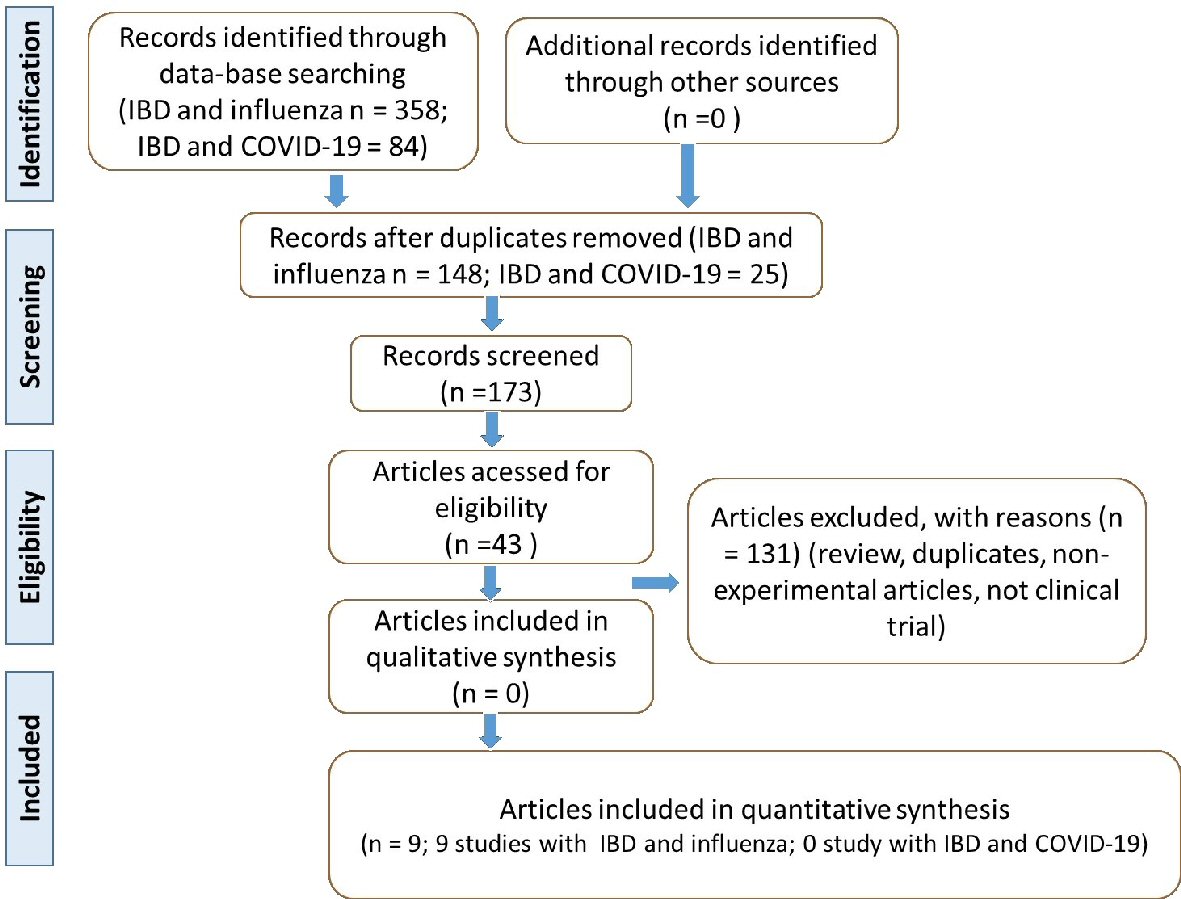
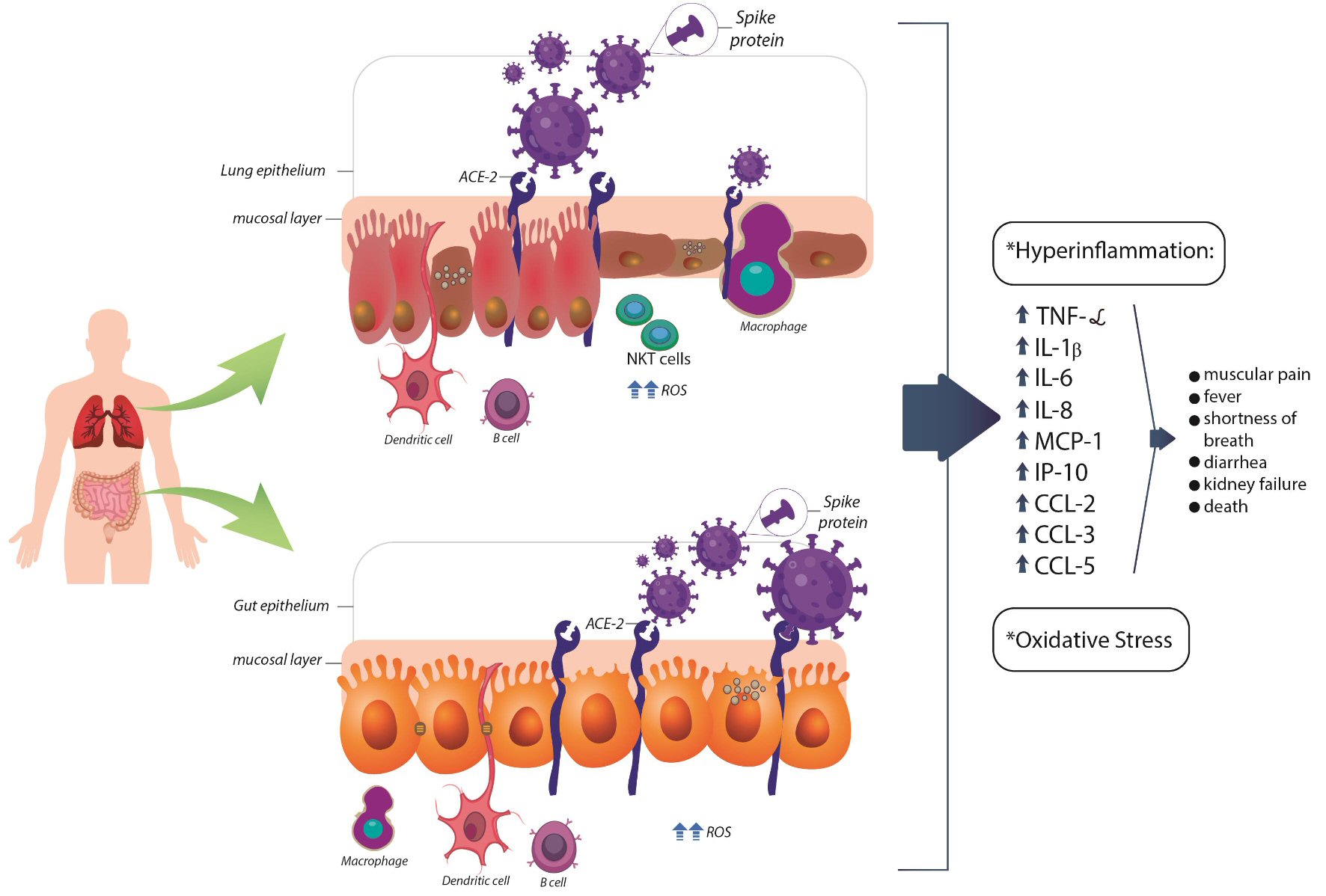
Figure 1. Flow diagram showing the study selection (PRISMA guidelines). IBD: inflammatory bowel disease; COVID-19: coronavirus disease 2019; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Review
Volume 14, Number 1, February 2021, pages 1-12
What Do Influenza and COVID-19 Represent for Patients With Inflammatory Bowel Disease?
Figures

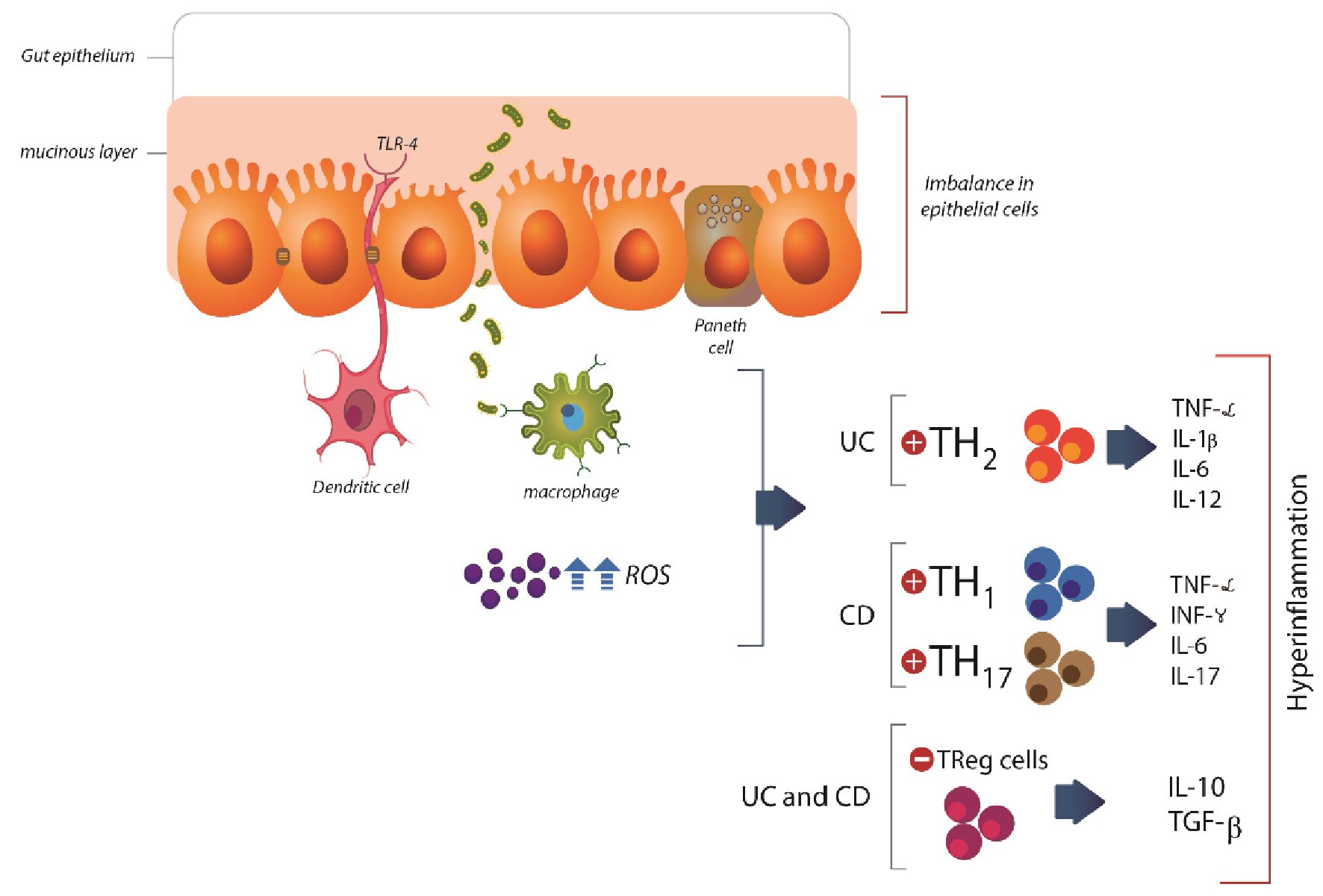
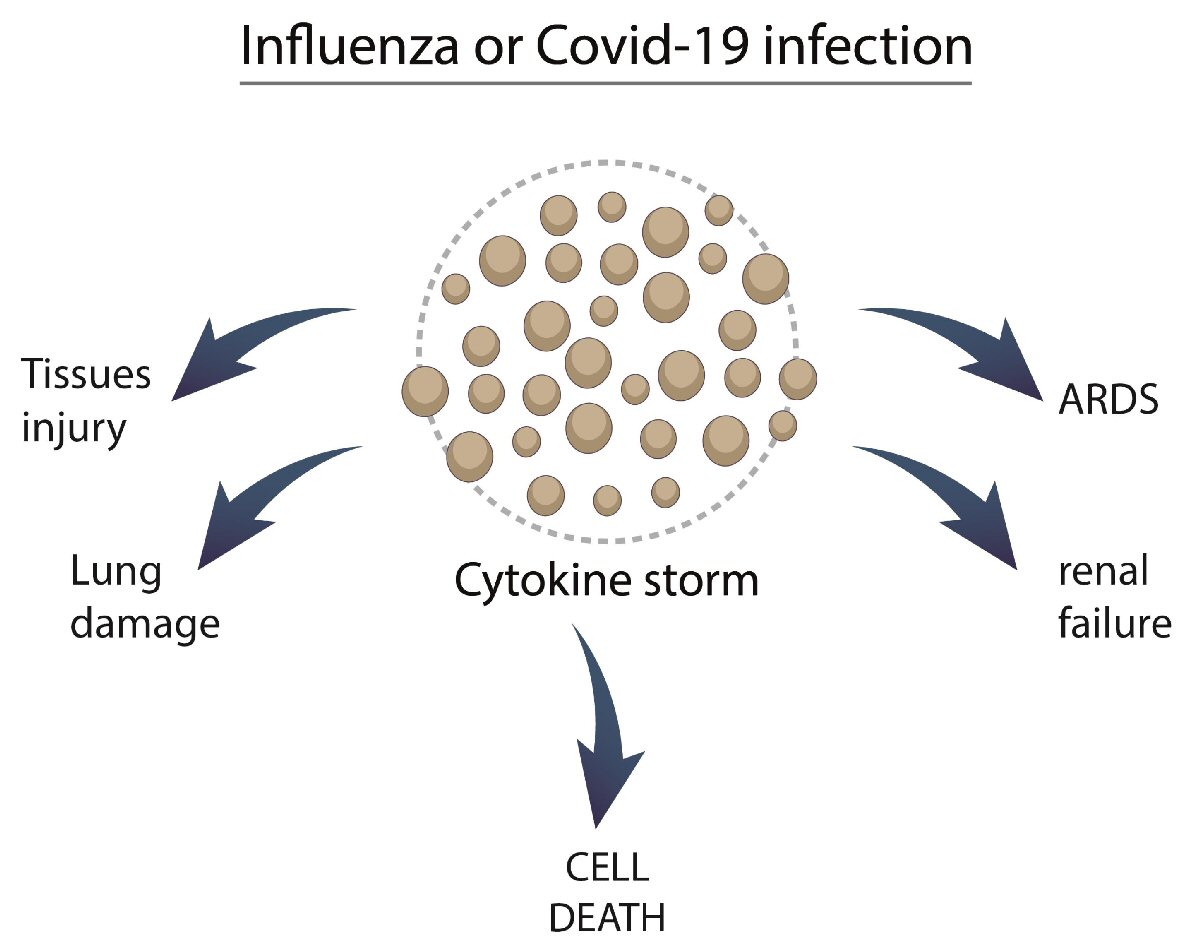
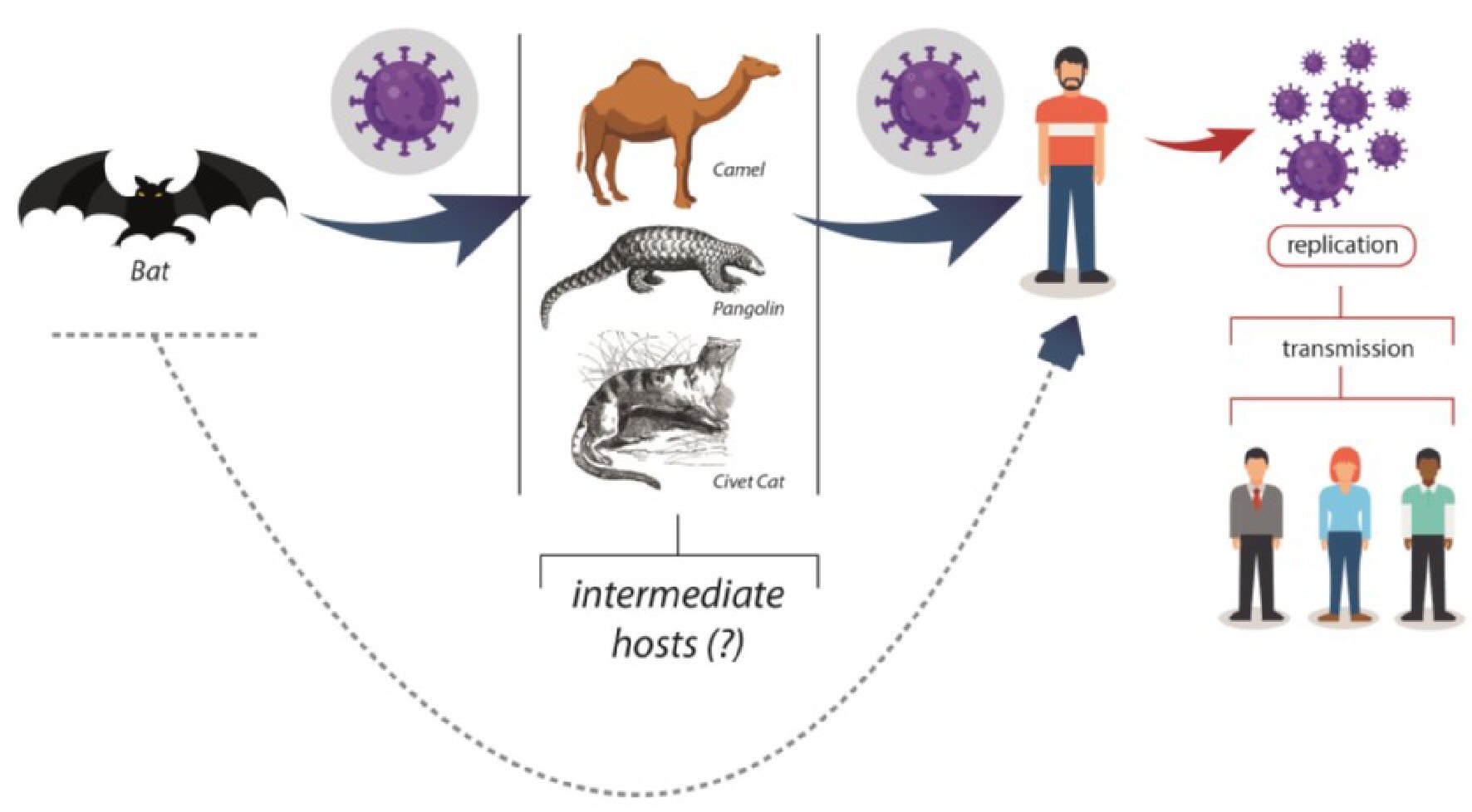
Table
| Reference | Country | Population | Intervention/comparison | Outcomes |
|---|---|---|---|---|
| Anti-HA: antihemagglutinin; Anti-NA: anti-neuraminidase; ASA: 5-acetylsalicylic acid; CD: Crohn’s disease; CDAI: Crohn’s disease activity index; HBI: Harvey Bradshaw index; IBD: inflammatory bowel disease; IL-2: interleukin 2; IM: intramuscular; IS: immunosuppressive; QIV: quadrivalent inactivated influenza vaccine; SC: subcutaneously; TNF: tumoral necrosis factor; UC: ulcerative colitis; HC: health control; yrs: years. | ||||
| [18] | USA | 69 IBD patients (40 on anti-TNF monotherapy, 19 on vedolizumab), and 20 healthy controls (HC) (18 - 64 yrs). | Patients were allocated to receive influenza high dose vaccine or standard dose. | IBD patients on anti-TNF monotherapy that received a high-dose influenza vaccine showed significantly increased post-immunization antibody levels compared with the standard vaccine. |
| [26] | Japan | 44 CD and 88 UC (41-42 yrs) with no significant differences in immunosuppressive therapies, disease activity, and endoscopic findings. | Patients and controls received single or double doses of seasonal QIV. 22 subjects received immunomodulatory monotherapy, 16 received anti-TNF-α single-agent therapy; 15 received immunosuppressant and anti-TNF-α agent. | Single-dose of QIV induced sufficient immunogenicity in IBD patients, and the additional vaccination did not show improvements. Immunogenicity was reduced in patients that were receiving infliximab. |
| [25] | Canada | 115 CD, 22 UC and 1 unclassified IBD (9 - 60 yrs) on the maintenance of infliximab therapy. | Patients received 2012/2013 influenza vaccine at the time of infliximab infusion or midway between infusions. | Serologic protection to the influenza vaccine was achieved in 45-80% of IBD patients on maintenance infliximab therapy. The vaccine timing relative to infliximab infusion did not affect the achievement of serologic protection. |
| [24] | France | 172 CD and 83 UC (18 - 64 yrs) who received at least 3 months of treatment (for those with IS or anti-TNF therapy) or without any indication to start IS therapeutics in the following 3 months. | Patients received the trivalent influenza vaccine for years 2009 - 2010 and 2010 - 2011. Hemagglutination inhibition titers were assessed before, 3 weeks, and 6 months after vaccination. Participants were divided into three groups: patients that had no IS, IS without anti-TNF, and anti-TNF with or without IS. | 3 weeks after the first vaccination, the rates of seroprotection were 77%, 75% and 66% for A/H1N12007, 77%, 68% and 52% for A/H3N2 and 97%, 96% and 95% for B strain in groups A, B, and C, respectively. Seroconversion rates for A/H1N12007, A/H3N2, and B strain did not differ according to treatment group. After 6 months of vaccination, seroprotection rates were lower in group C compared to group A, and B. Persistence of seroprotection was lower in patients with anti-TNF. |
| [23] | Japan | 38 CD, 33 UC, and 7 Behcet’s disease; (≥ 20 yrs) receiving IS therapy, immunomodulators and/or anti-TNF-a agents, and 11 HC. | The participants received the trivalent influenza vaccine and randomized into groups of single vaccination and two vaccination booster. | No significant differences were seen in the immune response between 3 weeks post-vaccination in the single vaccination group and 3 weeks post-second vaccination in the booster vaccination group. A higher pre-vaccination titer was related to enough seroprotection rate after vaccination for the H1N1 strain. The second booster of trivalent influenza vaccination did not improve the immune response. |
| [22] | Hungary | 127 CD and 82 UC patients (> 18 yrs) stable for more than 3 months, with no signs of activity and not requiring any treatment modification. | 156 patients received influenza vaccination and 53 patients did not (control group). The influenza vaccine used was: A/California/7/2009 (H1N1), A/Victoria/361/2011 (H3N2), B/Wisconsin/1/2010-like B/Hubei-Wujiagang/158/2009. The whole virion vaccine (Fluval AB) was given to 57, and split virion vaccine (IDFlu9) was given to 99 patients. | Post-immunization titers of both influenza subtypes were significantly increased after split virion vaccines compared to the control group and whole virion vaccine group. The antibody titers of influenza B also increased in the split virion vaccine group treated with anti-TNF-α therapy. The levels of IL-2 decreased after the intervention. Disease relapse was observed in only 10% of the patients and was more common in vaccinated patients. |
| [21] | Italy | 36 CD, 26 UC, and 31 HC (18 - 75 yrs). 47 patients were on anti-TNF-α monotherapy and 15 on anti-TNF-α combined with IS. | All the subjects received the MF59-adjuvanted H1N1 vaccine. | Seroprotective titers in UC and CD patients were comparable to HC. The seroconversion rate was lower than HC in IBD patients both on anti-TNF-α monotherapy or combined with IS. There was a suboptimal response to the vaccine in IBD patients on therapy with anti-TNF-α and IS compared to those on anti-TNF-α monotherapy and HC. |
| [20] | Belgium | 407 CD, 159 UC, and 9 indeterminate colitis patients (40.3 yrs) with stable IBD treated with immunomodulators and/or biological therapy. | Patients received influenza H1N1 adjuvanted and non-adjuvanted vaccines. The authors evaluated the risk of the flare of IBD after vaccination and disease activity. | After 4 weeks of intervention, the absence of flare was observed in 377 patients with CD (96.7%) and 151 with UC (95.6%). The risk of IBD flare appears to be not increased after H1N1 vaccination. |
| [19] | Poland | 30 IBD (not specified the type) patients (6 - 18 yrs) with previously diagnosed IBD and 34 HC. | Participants were divided into 3 groups: A, treated with ASA, metronidazole or ciprofloxacin; B, treated with ASA and immunomodulatory; and C, healthy children as control. All the subjects were vaccinated with the split type vaccine with the antigens of influenza strains: A/New Caledonia/20/99 (H1N1), A/California/7/04 (H3N2), and B/Shanghai/361/02. | Anti-HA and anti-NA 1 and 6 months post-vaccination were higher than baseline levels. In group A, the protection rate achieved the highest level for antigens A/H1N1 and B 6 months after vaccination. In group B, the highest protection rate was observed after 6 months. The response rate in group A remained the same 1 and 6 months post-vaccination, while in group B, the highest response rate was noted 6 months after the vaccine. |