| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Original Article
Volume 16, Number 6, December 2023, pages 289-306
Comparing the Efficacy and Safety of Adalimumab and Vedolizumab in Treating Moderate to Severe Crohn’s Disease and Ulcerative Colitis
Nooraldin Merzaa, Yusuf Nawrasb, Omar Saabc, Dushyant Singh Dahiyad, Zohaib Ahmede, Meghana Ranabothub, Safa Boujemaaf, Mona Hassane, Abdallah Kobeissye, Kirthi Lilleyg, h
aDepartment of Internal Medicine, University of Toledo, Toledo, OH, USA
bUniversity of Toledo College of Medicine and Life Sciences, Toledo, OH, USA
cDepartment of Internal Medicine, Cleavland Clinic, Cleavland, OH, USA
dDepartment of Gastroenterology, University of Kansas Medical Center, Kansas City, KS, USA
eDepartment of Gastroenterology, University of Toledo, Toledo, OH, USA
fBiotechnology Development, Institute Pasteur De Tunis, University De Tunis El Manar, Tunis, Tunisia
gDepartment of Gastroenterology, Wayne State University, Detroit, MI, USA
hCorresponding Author: Kirthi Lilley, Department of Gastroenterology, Wayne State University, Detroit, MI, USA
Manuscript submitted August 19, 2023, accepted October 14, 2023, published online November 3, 2023
Short title: ADA and VDZ in Treating CD and UC
doi: https://doi.org/10.14740/gr1664
| Abstract | ▴Top |
Background: Numerous patients with inflammatory bowel disease (IBD) do not respond to conventional or biological therapy. Adalimumab (ADA) and vedolizumab (VDZ), according to certain research, may be a useful alternative treatment for these people. The purpose of this study was to assess the effectiveness and safety of using ADA and VDZ to treat moderate to severe IBD: Crohn’s disease (CD) and ulcerative colitis (UC).
Methods: We searched PubMed, Medline, Web of Science, Scopus, the Cochrane Library, Embase, Google Scholar, CINAHL, Clinicaltrials.gov, and WHO trials registry (ICTRP). Randomized controlled trials (RCTs) comparing ADA or VDZ with placebo in participants with active CD or UC were included. The primary outcomes were the clinical response and remission at induction and maintenance phases and mucosal healing. The secondary outcome was the incidence of profound negative events. The research used Comprehensive Meta-Analysis version 3 (Biostat Inc., USA).
Results: Eighteen RCTs were incorporated, in which 11 studies described the usefulness and safeness of ADA or VDZ in CD patients, and seven studies investigated the efficacy and safety of ADA or VDZ in UC patients. The meta-analysis revealed that both ADA and VDZ treatments were superior to placebo for producing clinical remission and eliciting clinical response at induction and maintenance phases in individuals with moderately to severely active CD or UC. Interestingly, we found that ADA was superior to VDZ as first-line treatment for patients with CD, but not UC.
Conclusion: ADA and VDZ are effective and safe in CD and UC patients. However, RCTs of a larger number of patients are still required for better assessing the safety profile of ADA and VDZ.
Keywords: Inflammatory bowel disease; Crohn’s disease; Ulcerative colitis; Vedolizumab; Adalimumab; PRISMA
| Introduction | ▴Top |
Inflammatory bowel disease (IBD) is a chronic intestinal inflammation that develops in a genetically vulnerable person as a result of host-microbial interactions. IBDs are autoimmune diseases that are characterized by inflammation of both the small and large intestines and by immune system attacks on digestive system components [1]. The two classes of IBD, Crohn’s disease (CD) and ulcerative colitis (UC), both commonly start in early adulthood; however, they can start at any age, starting in early childhood [2]. Although CD and UC can develop at any age beginning in early infancy, they typically do so in early adulthood [3]. Both CD and UC have numerous extraintestinal symptoms outside of the gastrointestinal tract. While the illnesses can be identified in the majority of patients, in at least 10% of patients, the features are so similar that it is first impossible to distinguish between the two conditions [4]. Both conditions have a genetic propensity; they are both incurable and have a significant morbidity. And lastly, both raise the chance of colorectal cancer [5]. IBD has no known medicinal or surgical treatment options. Anti-inflammatory medications are used to treat the illness, which can help keep the disease in remission and dramatically lessen its symptoms.
For CD, clinical remission denotes a Crohn’s Disease activity index (CDAI) score under 150, reflecting an absence of primary symptoms like abdominal pain and diarrhea, with normalized inflammatory markers. In UC, it is gauged by a Mayo score of 0 - 2 or a simple clinical colitis activity index (SCCAI) score of ≤ 2, signifying normalized bowel habits without blood presence. Conversely, a clinical response in CD means a 100-point or more decrease in the CDAI score, indicating symptom improvement, while for UC, it is marked by a notable drop in the Mayo score and reduced rectal bleeding. Anti-inflammatory medications, including 5-aminosalicylic acid, and immunomodulators, like azathioprine, mercaptopurine, methotrexate, infliximab, adalimumab (ADA), certolizumab, vedolizumab (VDZ) and natalizumab, are used to treat the symptoms of IBD [6]. ADA is a completely human IgG1 monoclonal antibody against tumor necrosis factor-α (TNF-α) that has also been demonstrated to achieve the clinical remission and maintain clinical response in patients with active inflammatory CD and UC [7]. Despite the widespread use of TNF-α antibodies in clinical practice, a sizable minority of patients are unable to establish or sustain remission while receiving treatment [8]. Patients with IBD who had an insufficient response to, lost response to, or were intolerable to either conventional therapy or a TNF-α antibody may benefit from the use of VDZ, a humanized anti-α4b7 integrin monoclonal antibody [9]. VDZ’s gut-selective mechanism is thought to be safer than the anti-TNF-α antibodies currently used to treat IBD [10]. For the purpose of choosing a course of treatment, comparative clinical data comparing various treatments are crucial. Due to the lack of data directly comparing VDZ with ADA at this time, an indirect comparison may be an alternate way to investigate the relative efficacy of both biologicals. This systematic review and meta-analysis will synthesize the available evidence on the efficacy and safety of ADA and VDZ for the treatment of moderate to severe IBD.
| Materials and Methods | ▴Top |
This systematic review and meta-analysis adhered to the guidelines set by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). Additionally, our meta-analysis was conducted in alignment with the standards of A MeaSurement Tool to Assess systematic Reviews (AMSTAR) [11].
The meta-analysis research involves synthesizing and comparing data from multiple previously published studies rather than collecting primary, new data from human participants. Given that it is a secondary analysis of existing public data, and no further data are being collected directly from participants, there is no direct interaction or intervention with human subjects. As a result, Institutional Review Board (IRB) approval, which primarily ensures the protection of the rights and welfare of human research participants, is not usually required for network meta-analyses.
Search strategy
A comprehensive examination of databases such as PubMed, Medline, Web of Science, Scopus, the Cochrane Library, Embase, Google Scholar, CINAHL, Clinicaltrials.gov, and the WHO trials registry (ICTRP) was carried out from their inception to April 2023 to find suitable studies. The search encompassed these term combinations: “Adalimumab” OR “ADA” OR “Vedolizumab” OR “VZB” with “Inflammatory Bowel Disease” OR “ulcerative colitis” OR “Crohn’s disease” and also included “randomized controlled trial”. The bibliography sections of pertinent studies and overview papers were hand-reviewed to detect additional essential works. Two investigators separately executed this search procedure.
Inclusion and exclusion criteria
We screened relevant articles by title and abstract after removing duplicates. Studies were eligible for inclusion if they addressed the efficacy and safety of ADA or VDZ in patients with UC or CD. The full text of the remaining studies was then examined to confirm eligibility.
The inclusion criteria were as follows: 1) randomized controlled trials (RCTs) reporting the efficacy and safety of ADA or VDZ in patients with UC or CD; 2) participants of any age diagnosed with CD or UC, as defined by conventional clinical, radiological, endoscopic, or histological criteria; 3) interventions that involve ADA or VDZ versus placebo or a control therapy; 4) publications reporting sufficient data to establish statistical analysis; and 5) studies published as original articles. The exclusion criteria were as follows: 1) full text not electronically accessible; 2) publication in a language other than English; 3) observational studies, comments, letters, editorials, protocols, guidelines, and review papers; and 4) studies with insufficient outcome data.
Outcomes
The primary outcomes were the clinical response and remission at induction and maintenance phases as well as mucosal healing. Regarding CD, clinical remission is defined as a CDAI score of < 150 and clinical response is defined as a decrease in CDAI score from baseline by ≥ 70 points and by ≥ 100 points. Regarding UC, clinical remission is defined as Mayo score ≤ 2 with no individual sub-score exceeding 1 point and clinical response was defined as a decrease from baseline in the total Mayo score by at least 3 points and at least 30% with an accompanying decrease in rectal bleeding sub-score of at least 1 point or an absolute rectal bleeding sub-score of 0 or 1. Mucosal healing was defined as Mayo endoscopy sub-score of 0 or 1. Secondary outcome was the incidence of serious adverse events.
Data collection
Two independent authors retrieved information from the eligible articles following the application of inclusion and exclusion criteria. We collected the following information using standardized data sheet: 1) study ID (name of first author, year of publication), 2) location, 3) period, 4) design, 5) study phase, 6) name of trial, 7) population, 8) sample size, 9) intervention, 10) mean age, 11) male sex (%), 12) trial duration (weeks), and 13) outcomes. Characteristics of included studies are summarized in Table 1 [12-29].
 Click to view | Table 1. Characteristics of Included Studies |
Quality assessment of the studies
The methodological integrity of the selected trials was evaluated based on the Jadad scale, focusing on aspects such as randomization, blinding, and participant withdrawals in the studies [30]. The grading scale spans from 0 to 5 points. Reports of lower quality score 2 or below, while those of higher quality attain a score of 3 or more [31]. Methodological quality of included studies is shown in Table 2 [12-29].
 Click to view | Table 2. Methodological Quality of Included Studies |
Data analysis
The statistical evaluations were carried out using Comprehensive Meta-Analysis version 3 by Biostat Inc., USA. Based on the outcomes of the reviewed studies, ADA and VDZ were analyzed independently compared to placebos. Safety-related data were scrutinized from the safety population. Binary results, like clinical remission and clinical response, were gauged using the odds ratio (OR) alongside its 95% confidence intervals (CIs). Study variability was examined via the Cochrane Chi-squared test (Chi2) and the I2 inconsistency statistic. A P-value below 0.05 or an I2 of 50% and above was a sign of noteworthy variability. When studies showcased pronounced consistency, a fixed-effects model was utilized. However, in cases of evident variability, a random-effects model was adopted [32]. Given the limited number of studies available for each comparison, funnel plots were not used to investigate publication bias. The analysis of results adhered to the intention-to-treat approach.
| Results | ▴Top |
Identification of studies
The search of the database yielded 607 studies for review. From these, 401 abstracts seemed potentially suitable, leading to a full-text examination. Only 18 of these articles satisfied the criteria, and they were incorporated into this systematic review and meta-analysis. Figure 1 displays the PRISMA flow diagram.
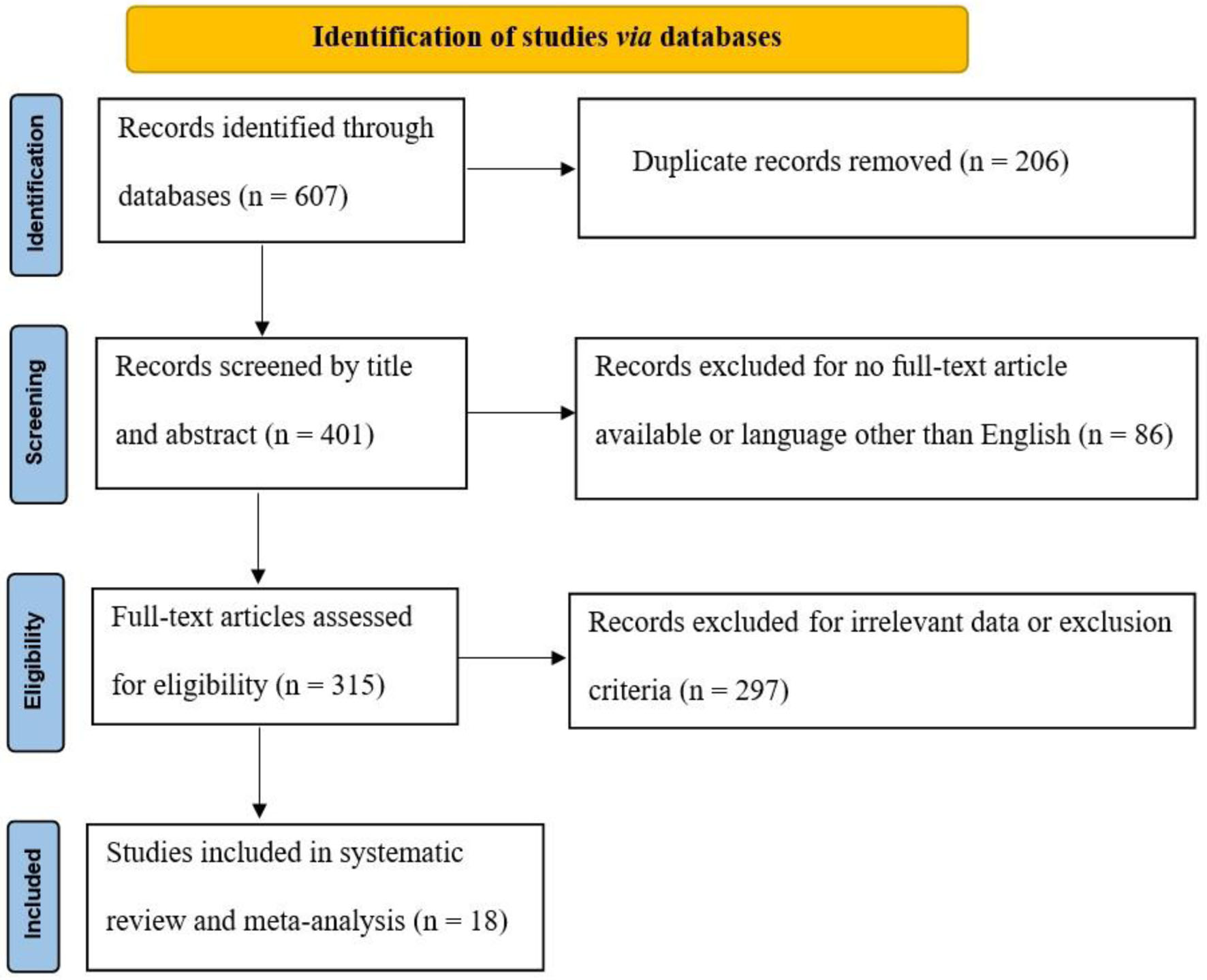 Click for large image | Figure 1. PRISMA flow diagram. PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses. |
Characteristics of included studies
The included articles were published between 2006 and 2023. Among the included studies, 11 and six studies investigated CD and UC, respectively. Among CD studies, seven and four studies reported the efficacy and safety of ADA and VZB, respectively. For the treatment of UC, only four and three studies described the efficacy of ADA and VZB, respectively. Among included studies, 5/18 studies reported induction phase, 3/18 studies described maintenance phase and 10 studies investigated both induction and maintenance phases. The sample size of the included articles varied from 55 to 115. The mean age of participants was ranged between 14.1 and 42.9 years. The majority of participants (> 50%) were male in 11 studies. Characteristics of included studies are summarized in Table 1.
Quality assessment
All RCTs were judged as being of high quality according to the Jadad scale (with 3 scores for eight studies and 4 scores for 10 studies). The reason for not receiving a full quality score was that the method of randomization and withdrawals/dropouts were not described.
Data analysis
Primary outcomes
1) CD
a) Induction phase
Clinical remission
The heterogeneity was low for both ADA (Chi2 = 9.579, P = 0.214, I2 = 26.92%) and VDZ (Chi2 = 0.443, P = 0.801, I2 = 0%) groups, so a fixed effect model was used. The forest plot analysis showed there was a significantly beneficial effect of ADA and VDZ for induction of remission with a superiority of ADA over VDZ (OR = 3.037, P = 0.000 and OR= 2.444, P = 0.000, respectively) (Fig. 2).
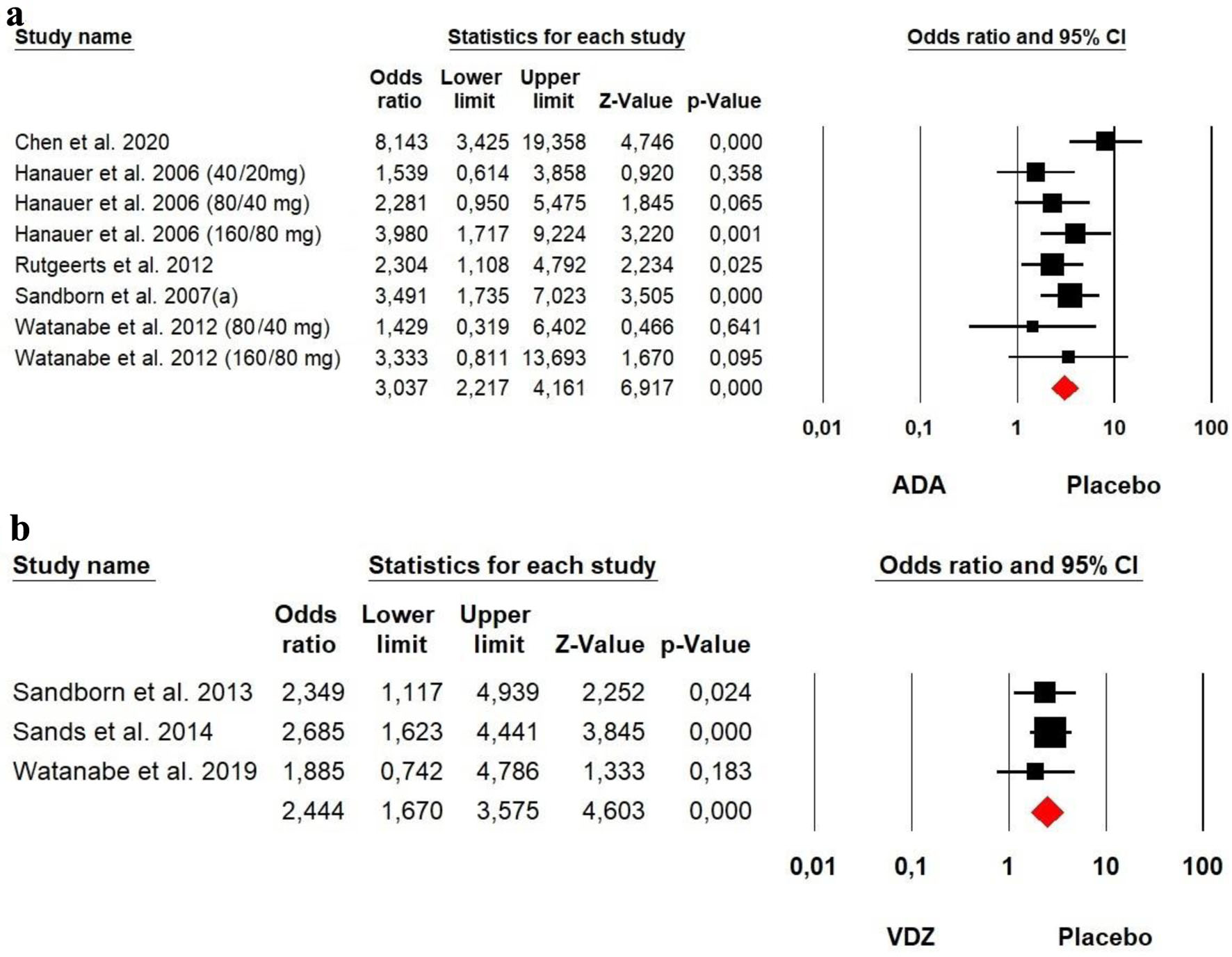 Click for large image | Figure 2. Forest plot for achieving clinical remission at induction phase in (a) ADA and (b) VDZ versus control group among CD patients. ADA: adalimumab; CD: Crohn’s disease; VDZ: vedolizumab. |
Clinical response
The heterogeneity was low for both ADA (Chi2 = 11.986, P = 0.101, I2 = 41.59%) and VDZ (Chi2 = 2.545, P = 0.280, I2 = 21.41%) groups, so a fixed effect model was used. The forest plot analysis showed there was a significantly beneficial effect of ADA and VDZ for induction of response with a slightly superiority of ADA over VDZ (OR = 2.601, P = 0.000 and OR = 2.254, P = 0.000, respectively) (Fig. 3).
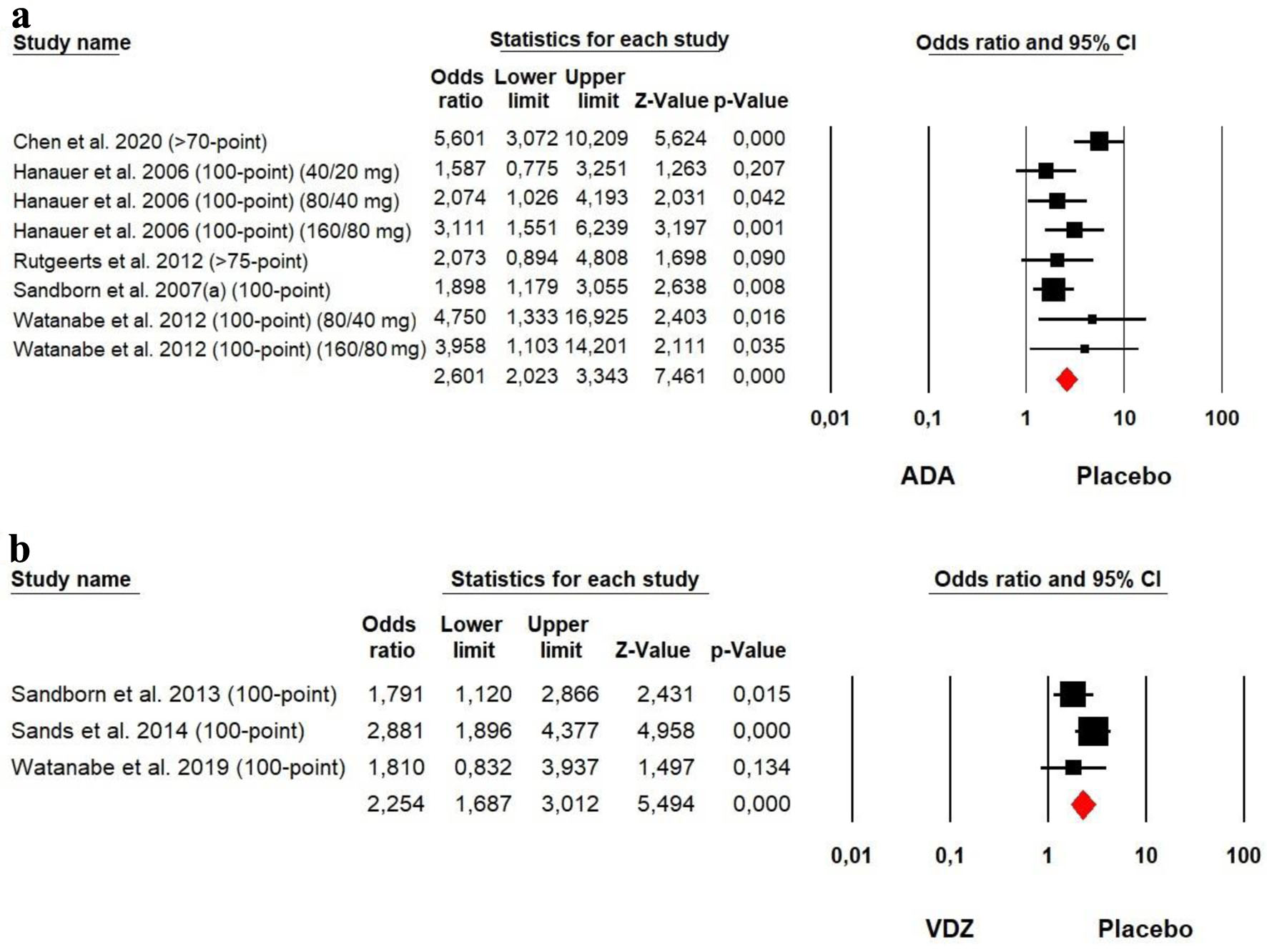 Click for large image | Figure 3. Forest plot for achieving clinical response at induction phase in (a) ADA and (b) VDZ versus control group among CD patients. ADA: adalimumab; CD: Crohn’s disease; VDZ: vedolizumab. |
b) Maintenance
Clinical remission
The heterogeneity was low for both ADA (Chi2 = 0.428, P = 0.995, I2 = 0%) and VDZ (Chi2 = 1.016, P = 0.602, I2 = 0%) groups, so a fixed effect model was used. Our meta-analysis on ADA and VDZ maintenance therapy showed that both ADA (OR = 4.808, P = 0.000) and VDZ (OR = 2.014, P = 0.000) were superior to the placebo in remission rates with a superiority of ADA over VDZ (Fig. 4).
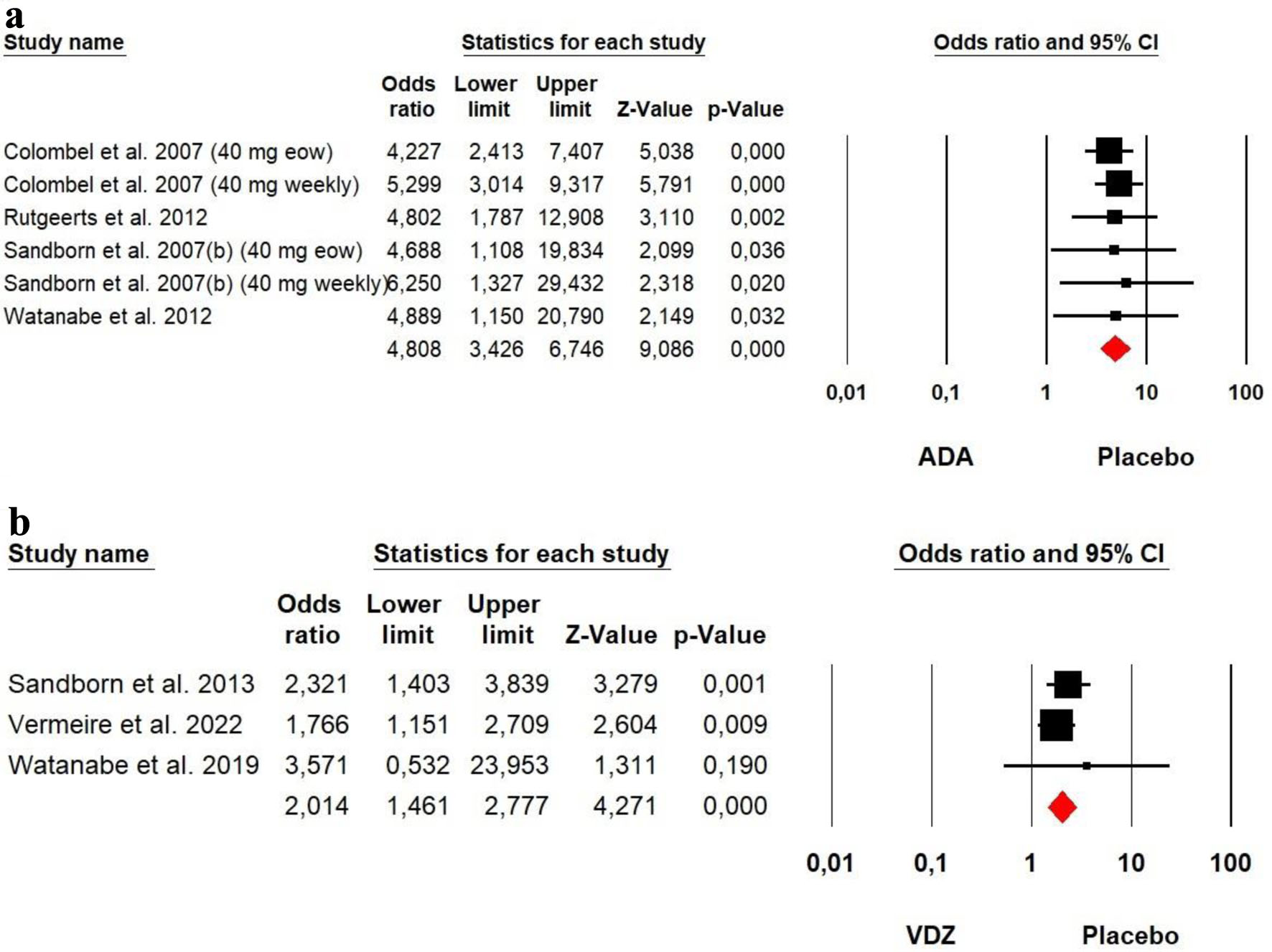 Click for large image | Figure 4. Forest plot for achieving clinical remission at maintenance phase in (a) ADA and (b) VDZ versus control group among CD patients. ADA: adalimumab; CD: Crohn’s disease; VDZ: vedolizumab. |
Clinical response
The heterogeneity was low for both ADA (Chi2 = 3.360, P = 0.645, I2 = 0%) and VDZ (Chi2 = 4.518, P = 0.104, I2 = 55.37%) groups, so a fixed effect model was used. Our meta-analysis on ADA and VDZ maintenance therapy showed that both ADA (OR = 4.330, P = 0.000) and VDZ (OR = 1.581, P = 0.004) were superior to the placebo in response rates with a superiority of ADA over VDZ (Fig. 5).
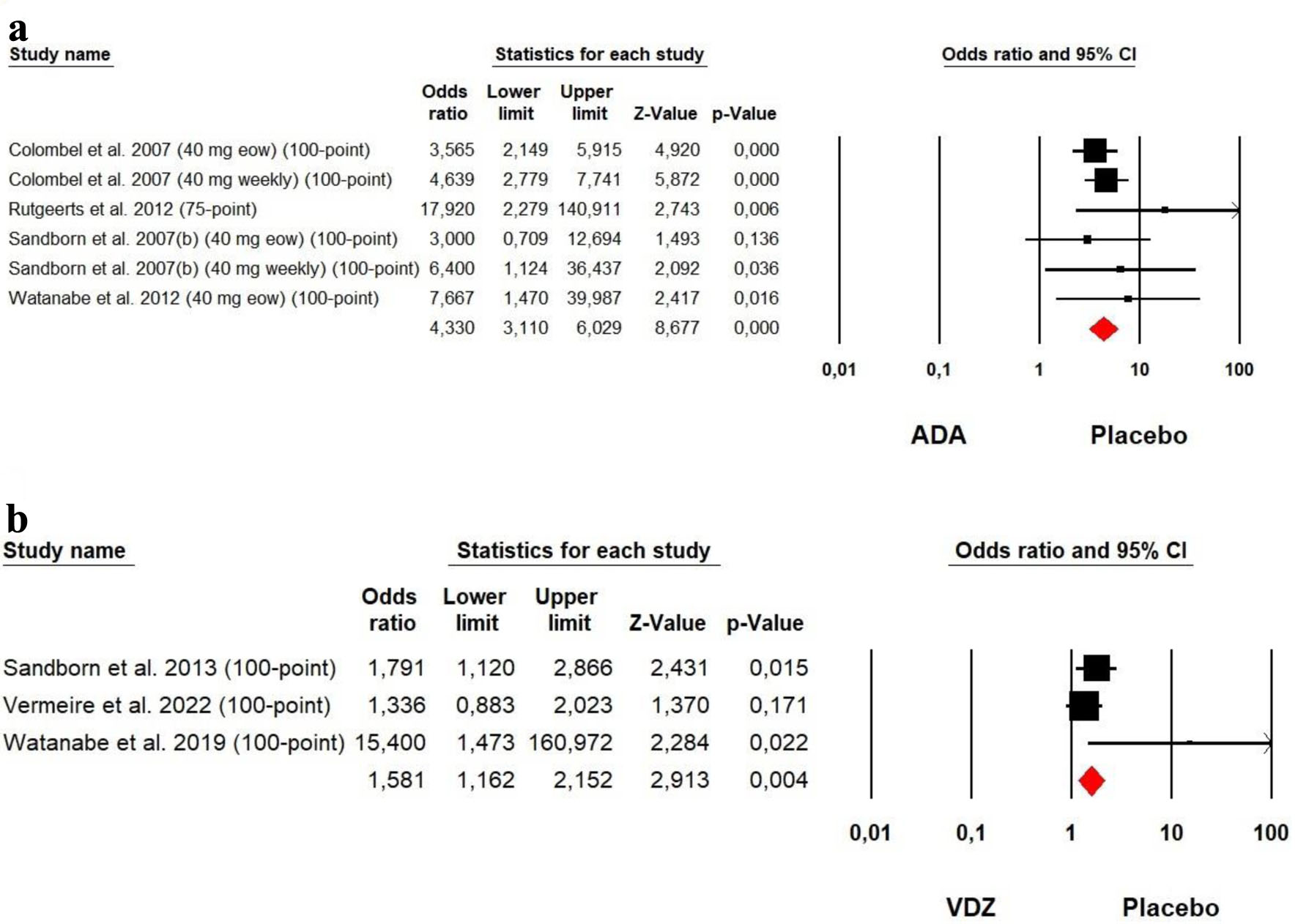 Click for large image | Figure 5. Forest plot for achieving clinical response at maintenance phase in (a) ADA and (b) VDZ versus control group among CD patients. ADA: adalimumab; CD: Crohn’s disease; VDZ: vedolizumab. |
2) UC
a) Induction phase
Clinical remission
The heterogeneity was low for both ADA (Chi2 = 8.389, P = 0.211, I2 = 28.48%) and VDZ (Chi2 = 2.002, P = 0.157, I2 = 50.03%) groups, so a fixed effect model was used. The forest plot analysis showed there was a significantly beneficial effect of ADA and VDZ for induction of remission with a superiority of VDZ over ADA (OR = 1.682, P = 0.001 and OR = 2.376, P = 0.002, respectively) (Fig. 6).
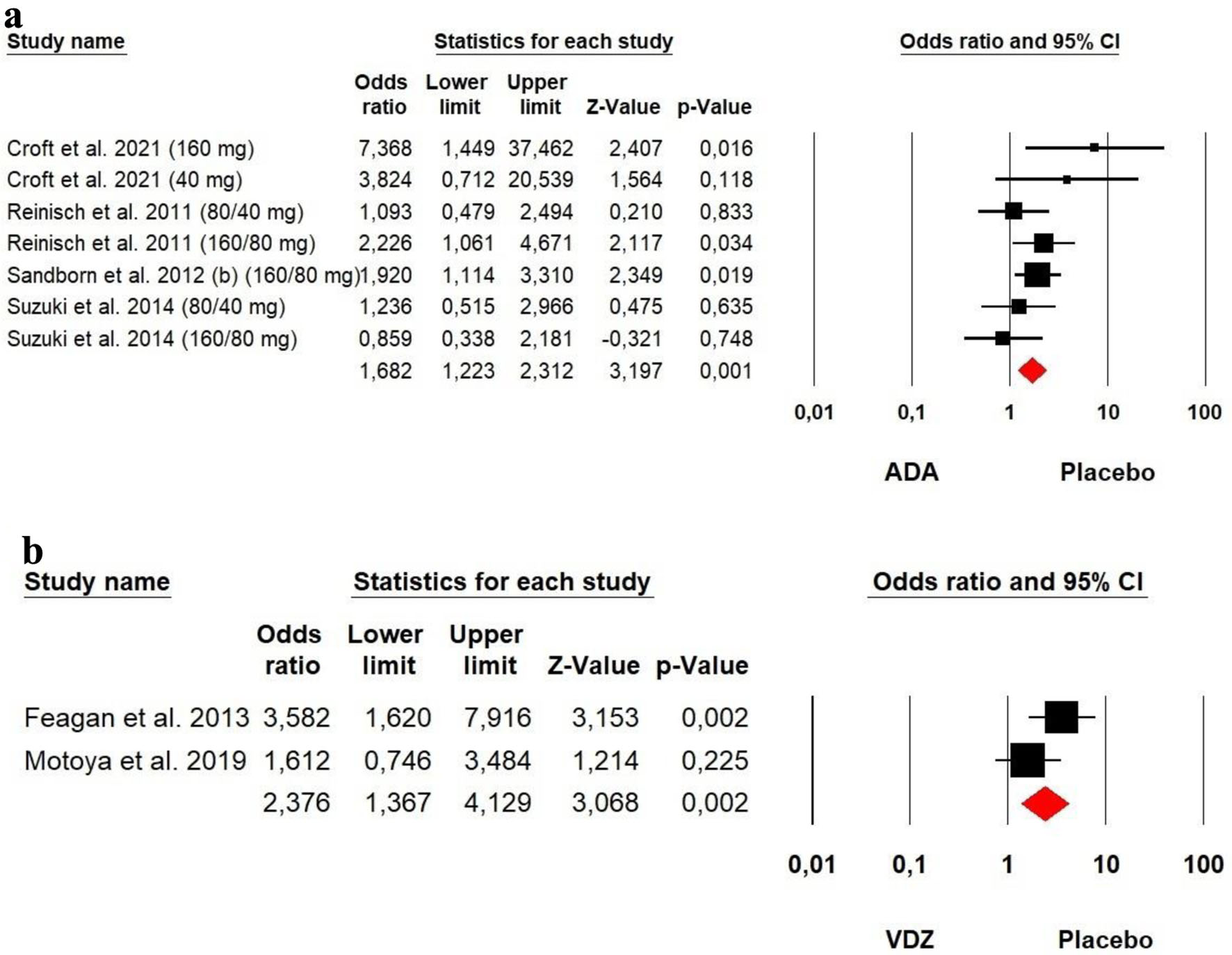 Click for large image | Figure 6. Forest plot for achieving clinical remission at induction phase in (a) ADA and (b) VDZ versus control group among UC patients. ADA: adalimumab; UC: ulcerative colitis; VDZ: vedolizumab. |
Clinical response
The heterogeneity was low for both ADA (Chi2 = 2.170, P = 0.705, I2 = 0%) and VDZ (Chi2 = 3.308, P = 0.069, I2 = 69.77%) groups, so a fixed effect model was used. The forest plot analysis showed there was a significantly beneficial effect of ADA and VDZ for induction of response with a slightly superiority of VDZ over ADA (OR = 1.616, P = 0.000 and OR = 1.998, P = 0.000, respectively) (Fig. 7).
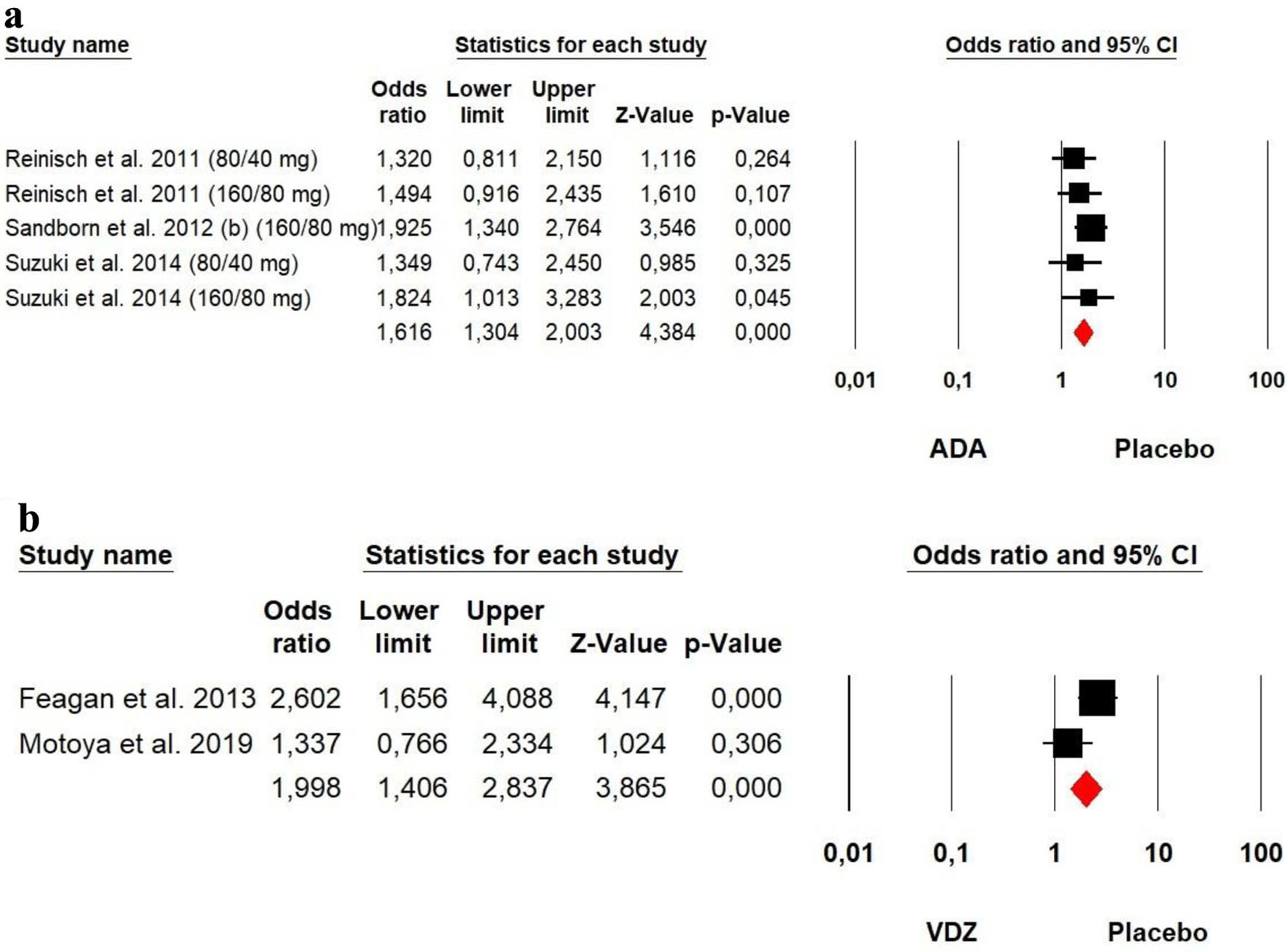 Click for large image | Figure 7. Forest plot for achieving clinical response at induction phase in (a) ADA and (b) VDZ versus control group among UC patients. ADA: adalimumab; UC: ulcerative colitis; VDZ: vedolizumab. |
Mucosal healing
The heterogeneity was high for ADA (Chi2 = 33.214, P = 0.000, I2 = 87.95%), so a random effect model was used. However, a low heterogeneity was detected for VDZ (Chi2 = 1.564, P = 0.211, I2 = 36.06%) groups, so a fixed effect model was used. The forest plot analysis showed there was a significantly beneficial effect of VDZ for induction of mucosal (OR = 1.744, P = 0.002). However, no significant difference was detected between ADA and placebo (OR = 1.039, P = 0.736) (Fig. 8).
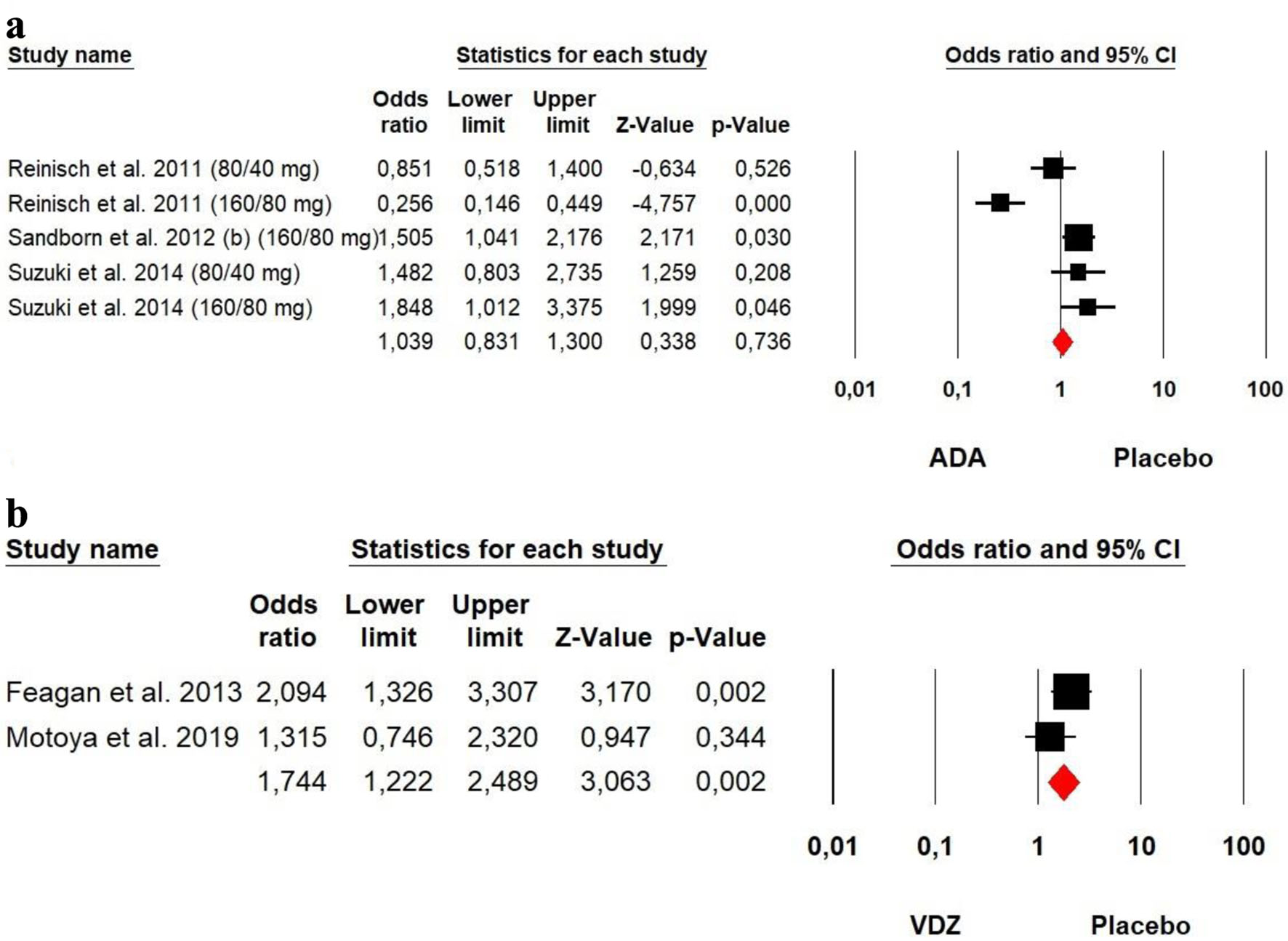 Click for large image | Figure 8. Forest plot for achieving mucosal healing at induction phase in (a) ADA and (b) VDZ versus control group among UC patients. ADA: adalimumab; UC: ulcerative colitis; VDZ: vedolizumab. |
b) Maintenance phase
Clinical remission
The heterogeneity was low for both ADA (Chi2 = 1.424, P = 0.700, I2 = 0%) and VDZ (Chi2 = 1.025, P = 0.906, I2 = 0%) groups, so a fixed effect model was used. Our meta-analysis on ADA and VDZ maintenance therapy showed that both ADA (OR = 2.675, P = 0.000) and VDZ (OR = 4.057, P = 0.000) were superior to the placebo in remission rates with a superiority of VDZ over ADA (Fig. 9).
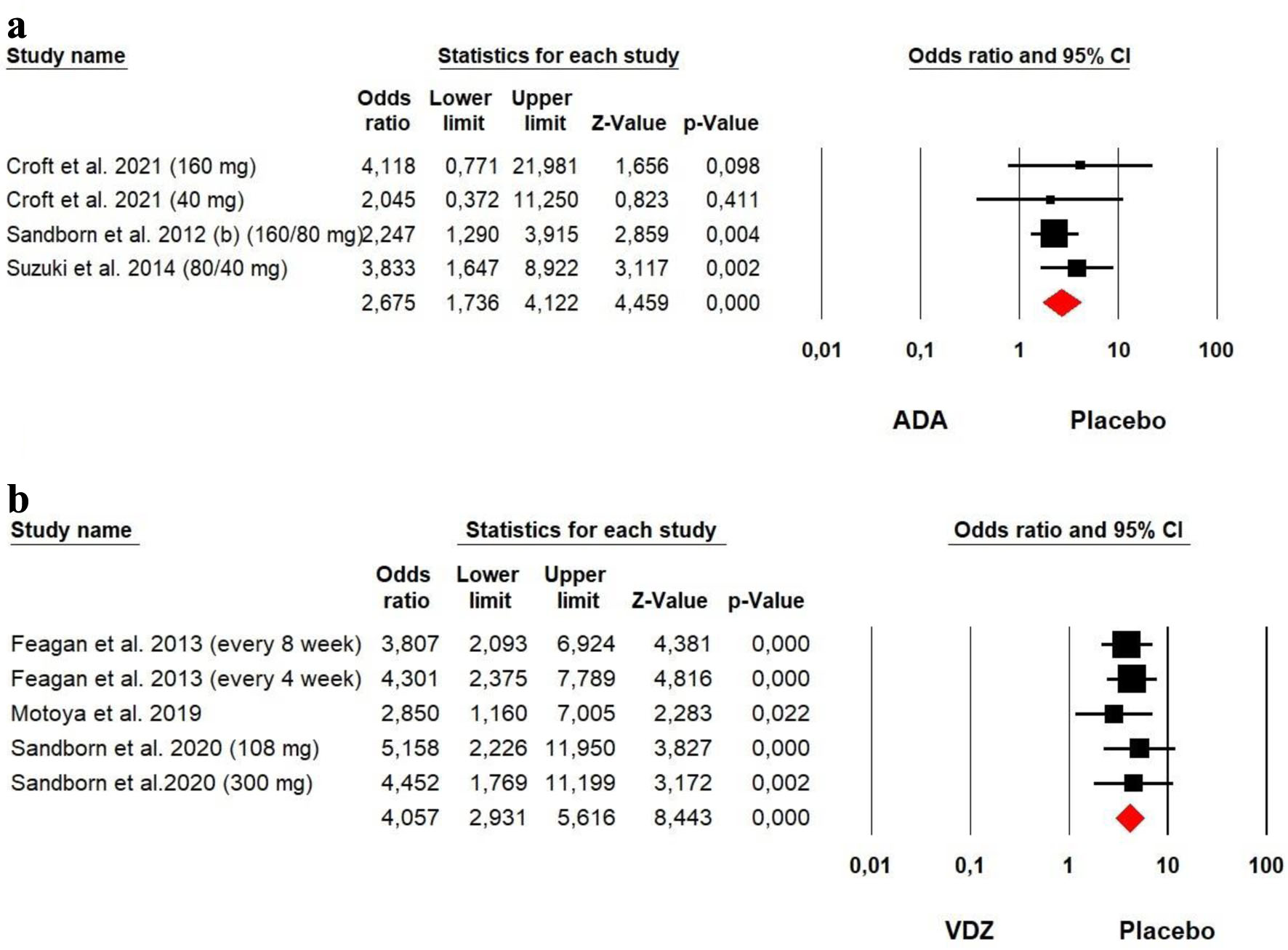 Click for large image | Figure 9. Forest plot for achieving clinical remission at maintenance phase in (a) ADA and (b) VDZ versus control group among UC patients. ADA: adalimumab; UC: ulcerative colitis; VDZ: vedolizumab. |
Clinical response
The heterogeneity was low for both ADA (Chi2 = 3.266, P = 0.352, I2 = 8.13%) and VDZ (Chi2 = 1.741, P = 0.783, I2 = 0%) groups, so a fixed effect model was used. Our meta-analysis on ADA and VDZ maintenance therapy showed that both ADA (OR = 2.191, P = 0.000) and VDZ (OR = 4.142, P = 0.000) were superior to the placebo in response rates with a superiority of VDZ over ADA (Fig. 10).
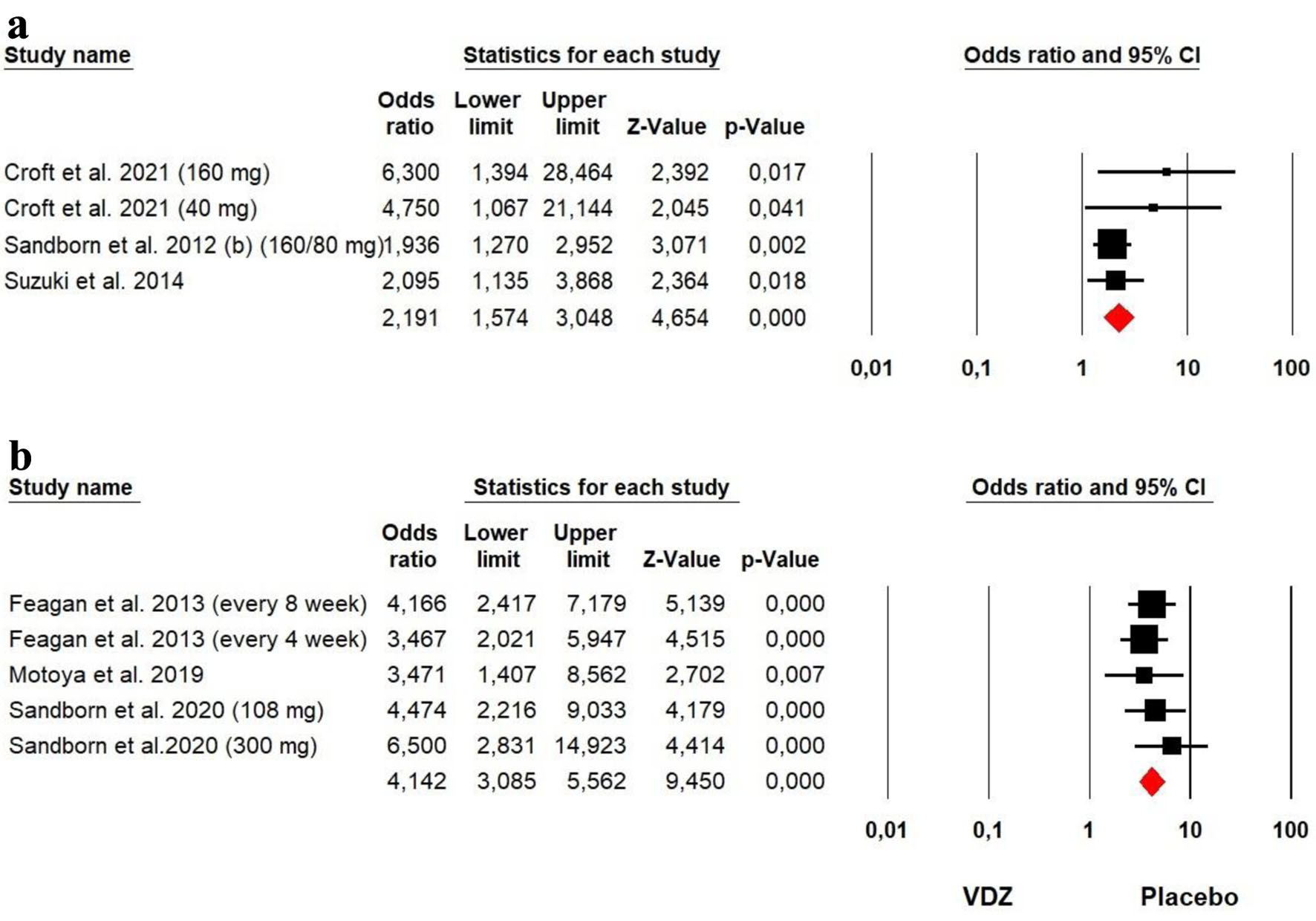 Click for large image | Figure 10. Forest plot for achieving clinical response at maintenance phase in (a) ADA and (b) VDZ versus control group among UC patients. ADA: adalimumab; UC: ulcerative colitis; VDZ: vedolizumab. |
Mucosal healing
The heterogeneity was low for both ADA (Chi2 = 1.801, P = 0.615, I2 = 0%) and VDZ (Chi2 = 0.291, P = 0.865, I2 = 0%) groups, so a fixed effect model was used. Our meta-analysis on ADA and VDZ maintenance therapy showed that both ADA (OR = 2.069, P = 0.000) and VDZ (OR = 4.216, P = 0.000) were superior to the placebo in mucosal healing rates with a superiority of VDZ over ADA (Fig. 11).
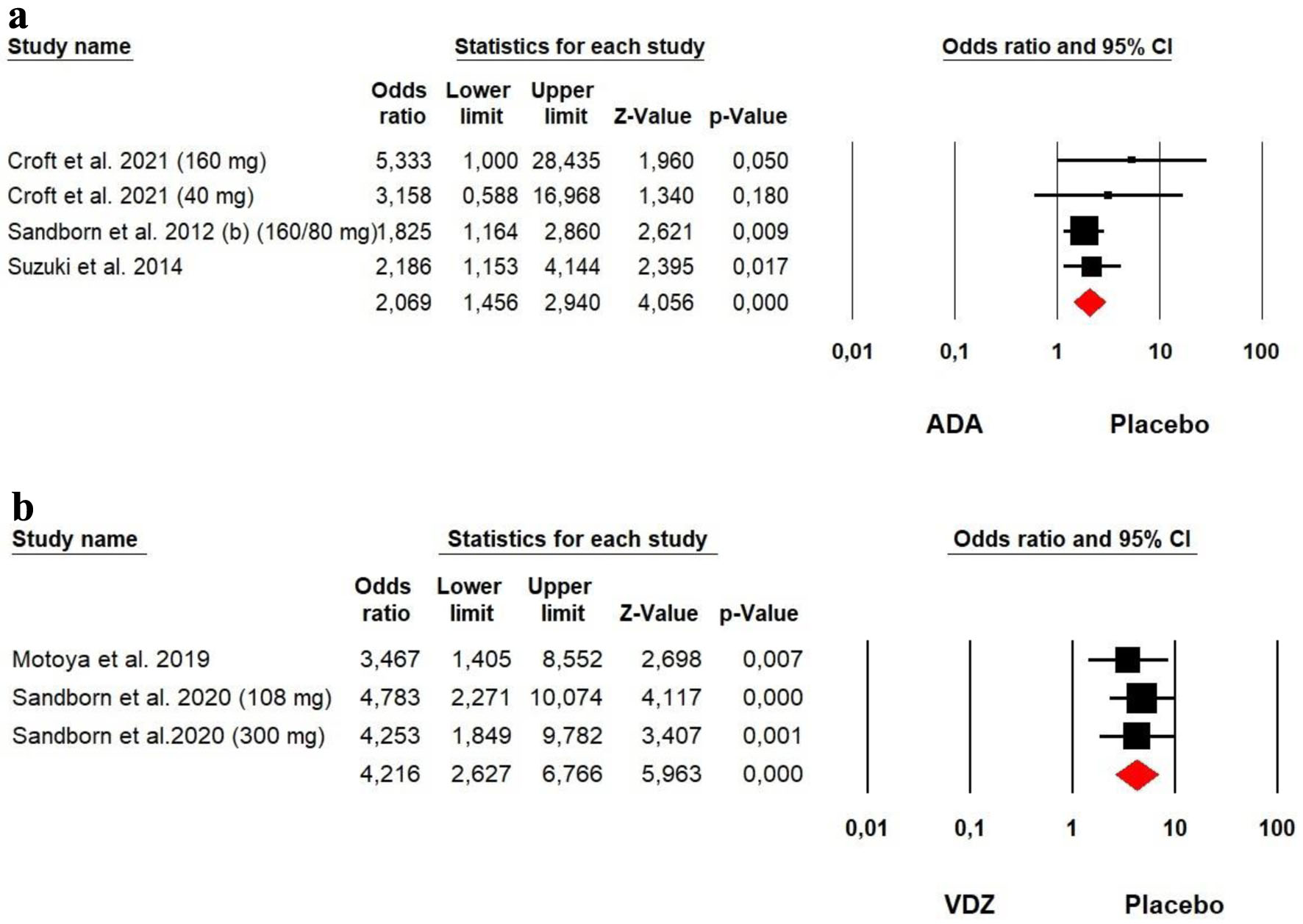 Click for large image | Figure 11. Forest plot for achieving mucosal healing at maintenance phase in (a) ADA and (b) VDZ versus control group among UC patients. ADA: adalimumab; UC: ulcerative colitis; VDZ: vedolizumab. |
Secondary outcomes: serious adverse events
1) CD
The heterogeneity was low for both ADA (Chi2 = 5.060, P = 0.887, I2 = 0%) and VDZ (Chi2 = 9.395, P = 0.052, I2 = 57.42%) groups, so a fixed effect model was used. Our meta-analysis showed that placebo presented more serious adverse events than ADA among CD patients (OR = 0.514, P = 0.000). However, no significant difference was detected between VDZ and placebo (OR = 1.284, P = 0.076) (Fig. 12).
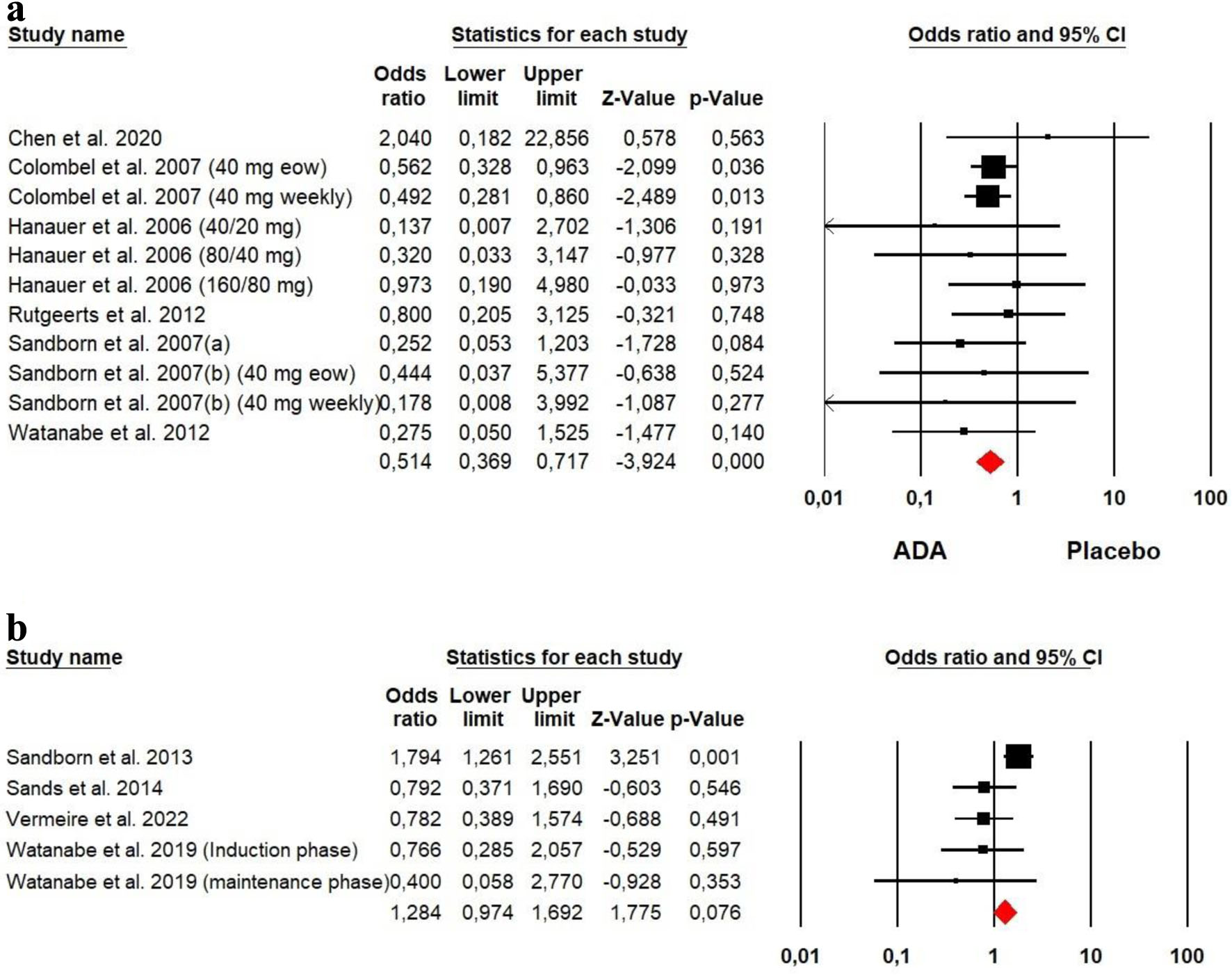 Click for large image | Figure 12. Forest plot for serious adverse events in (a) ADA and (b) VDZ versus control group among CD patients. ADA: adalimumab; CD: Crohn’s disease; VDZ: vedolizumab. |
2) UC
The heterogeneity was low for both ADA (Chi2 = 4.757, P = 0.190, I2 = 36.93%) and VDZ (Chi2 = 0.703, P = 0.951, I2 = 0%) groups, so a fixed effect model was used. Our meta-analysis showed that no significant difference was detected between ADA and VDZ versus placebo in terms of serious adverse events among UC patients (OR = 0.890, P = 0.512 and OR = 0.979, P = 0.904, respectively) (Fig. 13).
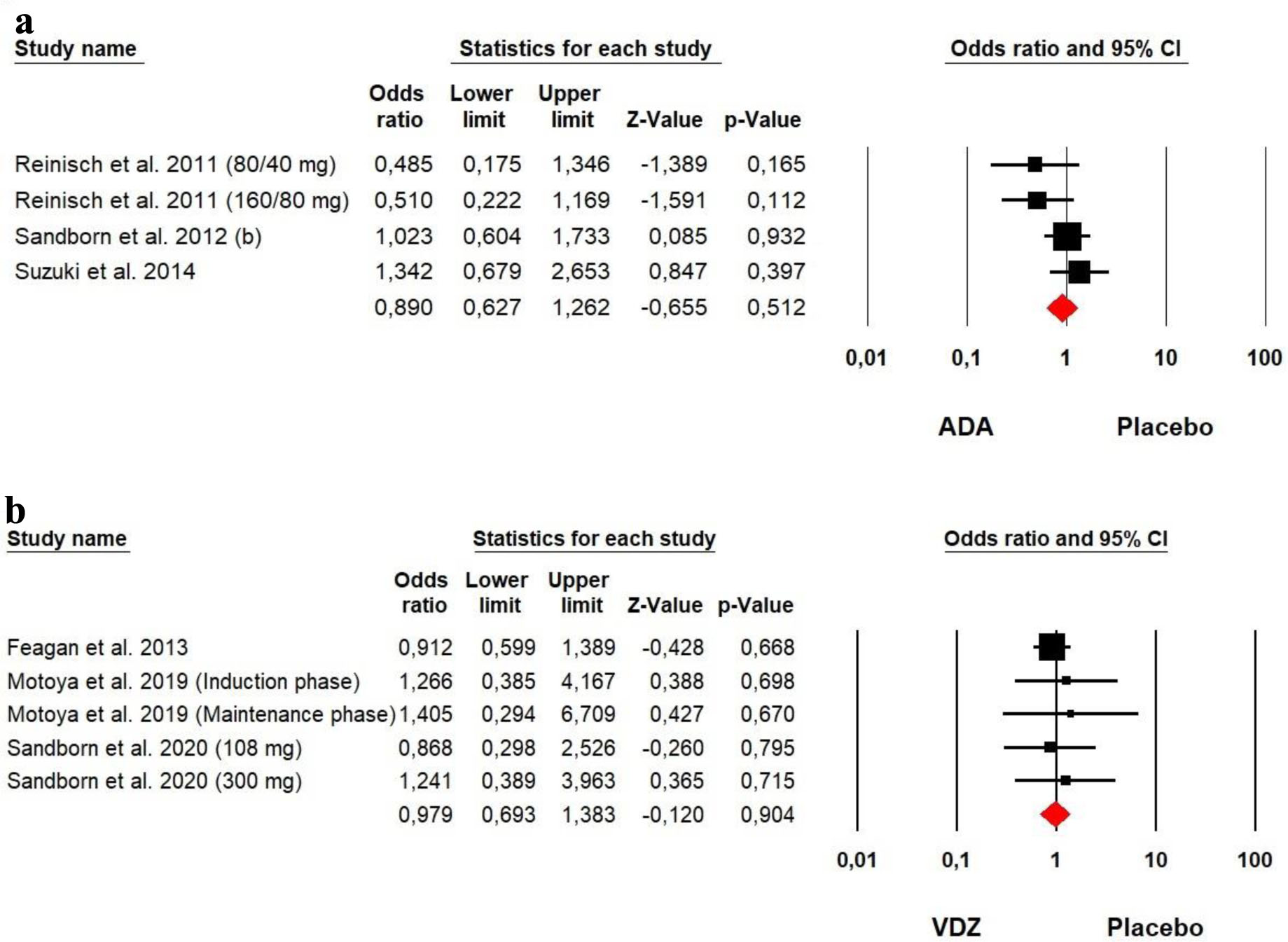 Click for large image | Figure 13. Forest plot for serious adverse events in (a) ADA and (b) VDZ versus control group among UC patients. ADA: adalimumab; UC: ulcerative colitis; VDZ: vedolizumab. |
| Discussion | ▴Top |
In this meta-analysis, we have shown that ADA treatment was superior to placebo for producing clinical remission and eliciting clinical response at induction phase in individuals with moderately to severely active CD. For example, participants in the biologic-naive CLASSIC I trial had a remission rate of 36% in the ADA group at induction phase compared to 12% in the placebo group [14]. Remission rates that were comparable were seen in the other investigations. The 160 mg/80 mg dose group is frequently utilized, and it was shown that it may be most beneficial in bringing about clinical remission and a clinical response [14, 18]. ADA was also investigated in four studies to maintain remission and clinical response in CD patients. These studies evaluated the long-term effectiveness of ADA [14, 15, 17, 18]. Both ADA groups (40 mg weekly and 40 mg every other week) exhibited significantly greater efficacy in the maintenance of clinical remission and response than the placebo group. The overall incidence of serious adverse events in ADA group during double-blind period was lower than that in the placebo group, and the overall safety profile observed was similar to those observed among other studies [33]. There are five further systematic review and meta-analysis studies that evaluated the effectiveness and security of ADA in CD patients. All of these reviews reached the same conclusion as revealed by us and showed that ADA was effective and significantly improved the life quality of CD participants [34-38].
VDZ is currently used in an emerging group of patients with IBD due to high efficacy and good safety profile. In this systematic review and meta-analysis of four RCTs of VDZ treatment in adults with CD, we made several key observations. First, we confirmed that VDZ treatment was superior to placebo for producing clinical remission and eliciting clinical response at induction and maintenance phase in individuals with moderately to severely active CD [19-22]. Second, the overall incidence of serious adverse events in VDZ group was similar to that in the placebo group.
On the other side, there are four further systematic review and meta-analysis studies that assessed the efficacy of VDZ in CD patients. VDZ was found to have a favorable efficacy and safety profile in bio-naive patients with CD, as reported by Attauabi et al [39]. Chandar et al revealed that natalizumab and VDZ were effective in inducing remission and response in patients with CD, with similar efficacy in anti-TNF-naive and anti-TNF-exposed patients [40], while Peyrin-Biroulet demonstrated that infliximab had better efficacy in the induction phase and comparable efficacy during the maintenance phase and overall safety profile compared to VDZ [41]. In the same context, Parrot et al showed that ustekinumab and VDZ were similarly effective in induction, but as maintenance treatment, ustekinumab appears to be more effective than VDZ [42].
To the best of our knowledge, there are presently no effectiveness or safety profile comparisons between ADA and VDZ in CD patients, in the literature. The positioning of ADA and VDZ in the therapeutic paradigm of CD patients should be based on indirect comparisons for clinical efficacy (clinical response, induction and maintenance of remission), as well as for safety profile, due to the lack of direct clinical comparisons. In this meta-analysis, we showed that ADA was superior to VDZ for producing both clinical remission and response at induction phase. Similarly, ADA was proven to be more effective than VDZ in the maintenance of clinical remission and response. On the other hand, we revealed that VDZ presented more serious adverse events than ADA. All these findings reveal that ADA seems to be more effective than VDZ to treat CD patients. However, there are no previous studies that confirm this conclusion.
Regarding UC, meta-analysis studies about the effectiveness of ADA and VDZ are limited [43-45]. Our meta-analysis proved that both ADA and VDZ treatments were superior to placebo for producing clinical remission and eliciting clinical response at induction and maintenance phases in individuals with moderately to severely active UC [23, 25-27]. However, we revealed that VDZ was superior to ADA with respect to achievement of clinical remission and response as well as mucosal healing contrary to CD patients. However, no significant difference was detected between ADA and VDZ versus placebo in terms of serious adverse events among UC patients. Similar results have been mentioned by Sands et al. Indeed, a comparison between VDZ and ADA for moderate to severe UC showed that VDZ presented higher efficacy than ADA in terms of clinical remission and endoscopic improvement, but not corticosteroid-free clinical remission [46]. However, an indirect comparison between VDZ and ADA for biologic-naive patients with UC demonstrated that VDZ has comparable efficacy to ADA [45]. Additional well-designed RCTs are needed to confirm these results.
In our study, ADA demonstrated a superior efficacy in managing CD, particularly in induction of remission and mucosal healing. This suggests that patients with CD might derive greater benefits from ADA, especially in cases resistant to conventional therapies. On the other hand, VDZ exhibited pronounced effectiveness in UC patients, showing better maintenance of remission and a more favorable safety profile. This implies that for UC patients, especially those with moderate to severe forms or those who have previously failed other biological treatments, VDZ might be a more suitable therapeutic option. Clinicians and researchers should consider these differential impacts when choosing the most appropriate treatment for CD and UC.
This research has its constraints. Since the meta-analysis relied on data from published works, there is a chance that publication bias might have led to underrepresentation of non-significant findings. Moreover, undertaking a meta-analysis on ADA and VDZ poses challenges because of dose differences. The limited quantity of studies further exacerbated the problem. These constraints impeded the direct comparison of varied research findings, thereby complicating the aggregated analysis and contributing to discrepancies in the meta-analysis. As such, the inherent variability typical of meta-analysis studies can influence the interpretation of outcomes [47]. As a result, careful consideration must be given to the present work’s findings.
Conclusion
Although there is no known treatment for IBD, there is now enough proof that a number of pharmacological substances can reduce intestinal inflammation. Our meta-analysis suggests that both ADA and VDZ are superior to placebo for induction and maintenance of clinical remission and response in patients with moderately to severely active CD and UC. Also, we noticed that serious adverse events were lower in ADA and VDZ participants compared with placebo. The low number of events raises questions about the impact of ADA and VDZ on serious adverse events. Therefore, no definitive conclusions about the safety of ADA and VDZ can be made. According to our findings, ADA seems to be superior to VDZ in CD patients, while VDZ has better efficacy compared to ADA in UC patients. Further studies, prospective, longer duration, with more participants are required to assess the long-term efficacy and safety of ADA in CD participants, and future RCTs should more clearly assess the serious adverse events.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Not applicable.
Author Contributions
Manuscript writing and overview of published works: Kirthi Lilley, Mona Hassa, Nooraldin Merza, Omar Saab, Dushyant Singh Dahiya, and Safa Boujemaa. Database extraction, results, and statistics: Nooraldin Merza, Zohaib Ahmed, Yusuf Nawras, Dushyant Singh Dahiya, Kirthi Lilley, and Meghana Ranabothu. Manuscript preparation and editing: Mona Hassa, Nooraldin Merza, Zohaib Ahmed, Yusuf Nawras, Omar Saab, Meghana Ranabothu, and Abdallah Kobeissy. Final drafting, review, and approval: Nooraldin Merza, Kirthi Lilley, Abdallah Kobeissy, Safa Boujemaa, and Mona Hassan.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
ADA: adalimumab; CD: Crohn’s disease; IBD: inflammatory bowel disease; IRB: Institutional Review Board; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses; UC: ulcerative colitis; VDZ: vedolizumab
| References | ▴Top |
- Fakhoury M, Negrulj R, Mooranian A, Al-Salami H. Inflammatory bowel disease: clinical aspects and treatments. J Inflamm Res. 2014;7:113-120.
doi pubmed pmc - Wehkamp J, Gotz M, Herrlinger K, Steurer W, Stange EF. Inflammatory bowel disease: Crohn’s disease and ulcerative colitis. Dtsch Arztebl International. 2016;113:72-82.
doi - Gryboski JD. Ulcerative colitis in children 10 years old or younger. J Pediatr Gastroenterol Nutr. 1993;17(1):24-31.
doi pubmed - Seyedian SS, Nokhostin F, Malamir MD. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J Med Life. 2019;12(2):113-122.
doi pubmed pmc - de Campos Silva EF, Baima JP, de Barros JR, Tanni SE, Schreck T, Saad-Hossne R, Sassaki LY. Risk factors for ulcerative colitis-associated colorectal cancer: A retrospective cohort study. Medicine (Baltimore). 2020;99(32):e21686.
doi pubmed pmc - Burger D, Travis S. Conventional medical management of inflammatory bowel disease. Gastroenterology. 2011;140(6):1827-1837.e1822.
doi pubmed - Tursi A, Mocci G, Lorenzetti R, Allegretta L, Brandimarte G, Cassieri C, Colucci R, et al. Long-term real-life efficacy and safety of infliximab and adalimumab in the treatment of inflammatory bowel diseases outpatients. Eur J Gastroenterol Hepatol. 2021;33(5):670-679.
doi pubmed - Cherry LN, Yunker NS, Lambert ER, Vaughan D, Lowe DK. Vedolizumab: an alpha4beta7 integrin antagonist for ulcerative colitis and Crohn's disease. Ther Adv Chronic Dis. 2015;6(5):224-233.
doi pubmed pmc - Dassopoulos T, Cohen RD, Scherl EJ, Schwartz RM, Kosinski L, Regueiro MD. Ulcerative colitis care pathway. Gastroenterology. 2015;149(1):238-245.
doi pubmed - Soler D, Chapman T, Yang LL, Wyant T, Egan R, Fedyk ER. The binding specificity and selective antagonism of vedolizumab, an anti-alpha4beta7 integrin therapeutic antibody in development for inflammatory bowel diseases. J Pharmacol Exp Ther. 2009;330(3):864-875.
doi pubmed - Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
doi pubmed pmc - Chen B, Gao X, Zhong J, Ren J, Zhu X, Liu Z, Wu K, et al. Efficacy and safety of adalimumab in Chinese patients with moderately to severely active Crohn's disease: results from a randomized trial. Therap Adv Gastroenterol. 2020;13:1756284820938960.
doi pubmed pmc - Colombel JF, Sandborn WJ, Rutgeerts P, Enns R, Hanauer SB, Panaccione R, Schreiber S, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterology. 2007;132(1):52-65.
doi pubmed - Hanauer SB, Sandborn WJ, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh D, Panaccione R, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn's disease: the CLASSIC-I trial. Gastroenterology. 2006;130(2):323-333; quiz 591.
doi pubmed - Rutgeerts P, Van Assche G, Sandborn WJ, Wolf DC, Geboes K, Colombel JF, Reinisch W, et al. Adalimumab induces and maintains mucosal healing in patients with Crohn's disease: data from the EXTEND trial. Gastroenterology. 2012;142(5):1102-1111.e1102.
doi pubmed - Sandborn WJ, Rutgeerts P, Enns R, Hanauer SB, Colombel JF, Panaccione R, D'Haens G, et al. Adalimumab induction therapy for Crohn disease previously treated with infliximab: a randomized trial. Ann Intern Med. 2007;146(12):829-838.
doi pubmed - Sandborn WJ, Hanauer SB, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh DG, Panaccione R, et al. Adalimumab for maintenance treatment of Crohn's disease: results of the CLASSIC II trial. Gut. 2007;56(9):1232-1239.
doi pubmed pmc - Watanabe M, Hibi T, Lomax KG, Paulson SK, Chao J, Alam MS, Camez A, et al. Adalimumab for the induction and maintenance of clinical remission in Japanese patients with Crohn's disease. J Crohns Colitis. 2012;6(2):160-173.
doi pubmed - Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, Lukas M, et al. Vedolizumab as induction and maintenance therapy for Crohn's disease. N Engl J Med. 2013;369(8):711-721.
doi pubmed - Sands BE, Feagan BG, Rutgeerts P, Colombel JF, Sandborn WJ, Sy R, D'Haens G, et al. Effects of vedolizumab induction therapy for patients with Crohn's disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology. 2014;147(3):618-627.e613.
doi pubmed - Vermeire S, D'Haens G, Baert F, Danese S, Kobayashi T, Loftus EV, Bhatia S, et al. Efficacy and Safety of Subcutaneous Vedolizumab in Patients With Moderately to Severely Active Crohn's Disease: Results From the VISIBLE 2 Randomised Trial. J Crohns Colitis. 2022;16(1):27-38.
doi pubmed pmc - Watanabe K, Motoya S, Ogata H, Kanai T, Matsui T, Suzuki Y, Shikamura M, et al. Effects of vedolizumab in Japanese patients with Crohn's disease: a prospective, multicenter, randomized, placebo-controlled Phase 3 trial with exploratory analyses. J Gastroenterol. 2020;55(3):291-306.
doi pubmed pmc - Croft NM, Faubion WA, Jr., Kugathasan S, Kierkus J, Ruemmele FM, Shimizu T, Mostafa NM, et al. Efficacy and safety of adalimumab in paediatric patients with moderate-to-severe ulcerative colitis (ENVISION I): a randomised, controlled, phase 3 study. Lancet Gastroenterol Hepatol. 2021;6(8):616-627.
doi pubmed - Reinisch W, Sandborn WJ, Hommes DW, D'Haens G, Hanauer S, Schreiber S, Panaccione R, et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut. 2011;60(6):780-787.
doi pubmed - Sandborn WJ, van Assche G, Reinisch W, Colombel JF, D'Haens G, Wolf DC, Kron M, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012;142(2):257-265.e251-253.
doi pubmed - Suzuki Y, Motoya S, Hanai H, Matsumoto T, Hibi T, Robinson AM, Mostafa NM, et al. Efficacy and safety of adalimumab in Japanese patients with moderately to severely active ulcerative colitis. J Gastroenterol. 2014;49(2):283-294.
doi pubmed pmc - Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, Van Assche G, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369(8):699-710.
doi pubmed - Motoya S, Watanabe K, Ogata H, Kanai T, Matsui T, Suzuki Y, Shikamura M, et al. Vedolizumab in Japanese patients with ulcerative colitis: A Phase 3, randomized, double-blind, placebo-controlled study. PLoS One. 2019;14(2):e0212989.
doi pubmed pmc - Sandborn WJ, Baert F, Danese S, Krznaric Z, Kobayashi T, Yao X, Chen J, et al. Efficacy and safety of vedolizumab subcutaneous formulation in a randomized trial of patients with ulcerative colitis. Gastroenterology. 2020;158(3):562-572.e512.
doi pubmed - Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1-12.
doi pubmed - Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, Tugwell P, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet. 1998;352(9128):609-613.
doi pubmed - Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1(2):97-111.
doi pubmed - Colombel JF, Sandborn WJ, Reinisch W, Peyrin-Biroulet L, Panaccione R, Rutgeerts P, Hanauer SB, et al. Long-term safety of adalimumab in clinical trials in adult patients with Crohn's disease or ulcerative colitis. Aliment Pharmacol Ther. 2018;47(2):219-228.
doi pubmed - Abbass M, Cepek J, Parker CE, Nguyen TM, MacDonald JK, Feagan BG, Khanna R, et al. Adalimumab for induction of remission in Crohn's disease. Cochrane Database Syst Rev. 2019;2019(11):CD012878.
doi pubmed pmc - Huang ML, Ran ZH, Shen J, Li XB, Xu XT, Xiao SD. Efficacy and safety of adalimumab in Crohn's disease: meta-analysis of placebo-controlled trials. J Dig Dis. 2011;12(3):165-172.
doi pubmed - Singh S, Murad MH, Fumery M, Sedano R, Jairath V, Panaccione R, Sandborn WJ, et al. Comparative efficacy and safety of biologic therapies for moderate-to-severe Crohn's disease: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 2021;6(12):1002-1014.
doi pubmed pmc - Song YN, Zheng P, Xiao JH, Lu ZJ. Efficacy and safety of adalimumab for the Crohn's disease: a systematic review and meta-analysis of published randomized placebo-controlled trials. Eur J Clin Pharmacol. 2014;70(8):907-914.
doi pubmed - Yin J, Li Y, Chen Y, Wang C, Song X. Adalimumab for induction of remission in patients with Crohn's disease: a systematic review and meta-analysis. Eur J Med Res. 2022;27(1):190.
doi pubmed pmc - Attauabi M, Madsen GR, Bendtsen F, Seidelin JB, Burisch J. Vedolizumab as the first line of biologic therapy for ulcerative colitis and Crohn's disease - a systematic review with meta-analysis. Dig Liver Dis. 2022;54(9):1168-1178.
doi pubmed - Chandar AK, Singh S, Murad MH, Peyrin-Biroulet L, Loftus EV, Jr. Efficacy and safety of natalizumab and vedolizumab for the management of Crohn's disease: a systematic review and meta-analysis. Inflamm Bowel Dis. 2015;21(7):1695-1708.
doi pubmed - Peyrin-Biroulet L, Arkkila P, Armuzzi A, Danese S, Guardiola J, Jahnsen J, Lees C, et al. Comparative efficacy and safety of infliximab and vedolizumab therapy in patients with inflammatory bowel disease: a systematic review and meta-analysis. BMC Gastroenterol. 2022;22(1):291.
doi pubmed pmc - Parrot L, Dong C, Carbonnel F, Meyer A. Systematic review with meta-analysis: the effectiveness of either ustekinumab or vedolizumab in patients with Crohn's disease refractory to anti-tumour necrosis factor. Aliment Pharmacol Ther. 2022;55(4):380-388.
doi pubmed - Chen X, Hou J, Yuan Y, Huang C, Liu T, Mo C, Li H, et al. Adalimumab for moderately to severely active ulcerative colitis: a systematic review and meta-analysis. BioDrugs. 2016;30(3):207-217.
doi pubmed - Zhang ZM, Li W, Jiang XL. Efficacy and safety of adalimumab in moderately to severely active cases of ulcerative colitis: a meta-analysis of published placebo-controlled trials. Gut Liver. 2016;10(2):262-274.
doi pubmed pmc - Dignass AU, Siegmund B, Goertz R, Schneidewind G, Fanter L. Indirect comparison of vedolizumab and adalimumab for biologic-naive patients with ulcerative colitis. Scand J Gastroenterol. 2019;54(2):178-187.
doi pubmed - Sands BE, Peyrin-Biroulet L, Loftus EV, Jr., Danese S, Colombel JF, Toruner M, Jonaitis L, et al. Vedolizumab versus adalimumab for moderate-to-severe ulcerative colitis. N Engl J Med. 2019;381(13):1215-1226.
doi pubmed - Imrey PB. Limitations of Meta-analyses of Studies With High Heterogeneity. JAMA Netw Open. 2020;3(1):e1919325.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


