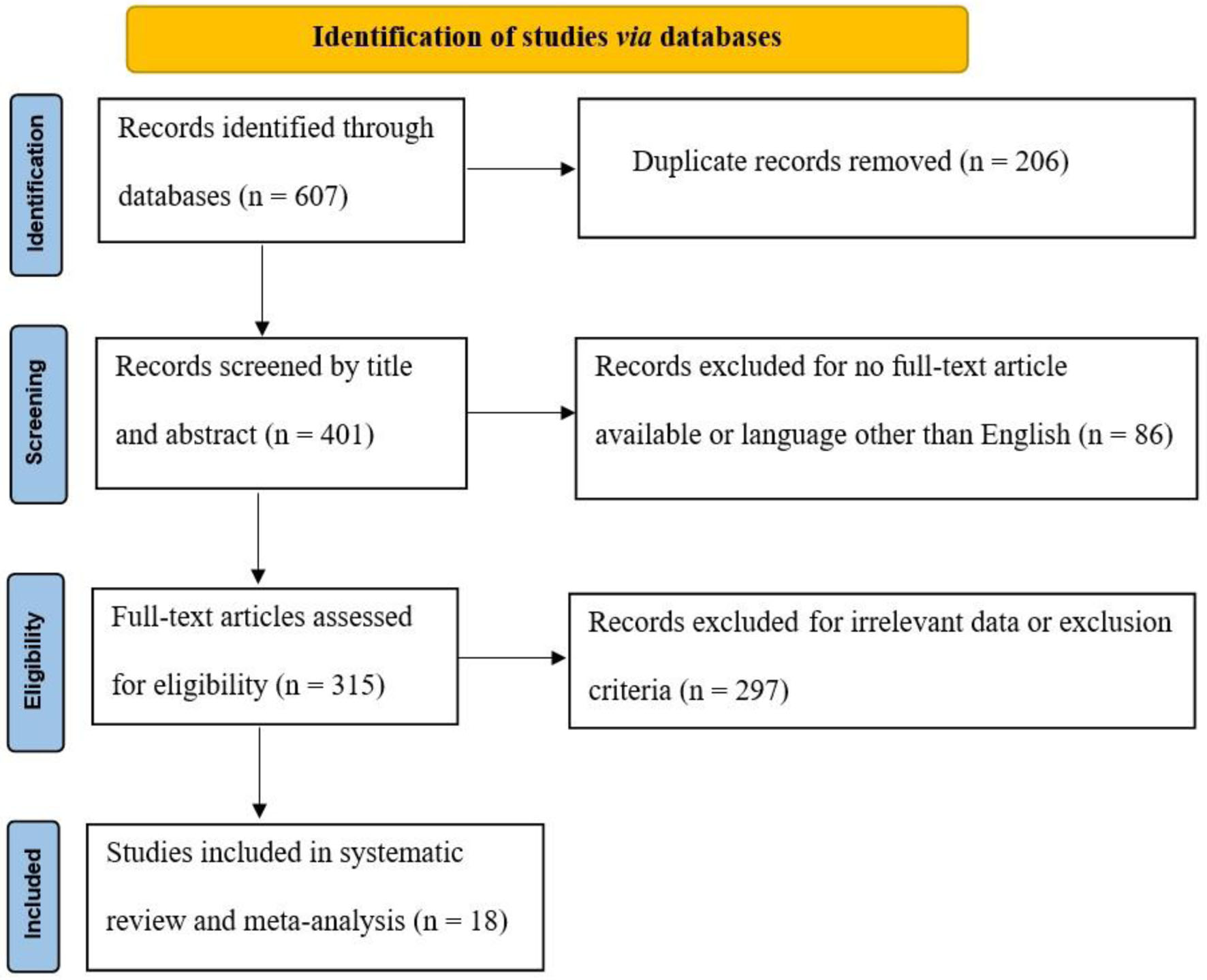
Figure 1. PRISMA flow diagram. PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Original Article
Volume 16, Number 6, December 2023, pages 289-306
Comparing the Efficacy and Safety of Adalimumab and Vedolizumab in Treating Moderate to Severe Crohn’s Disease and Ulcerative Colitis
Figures

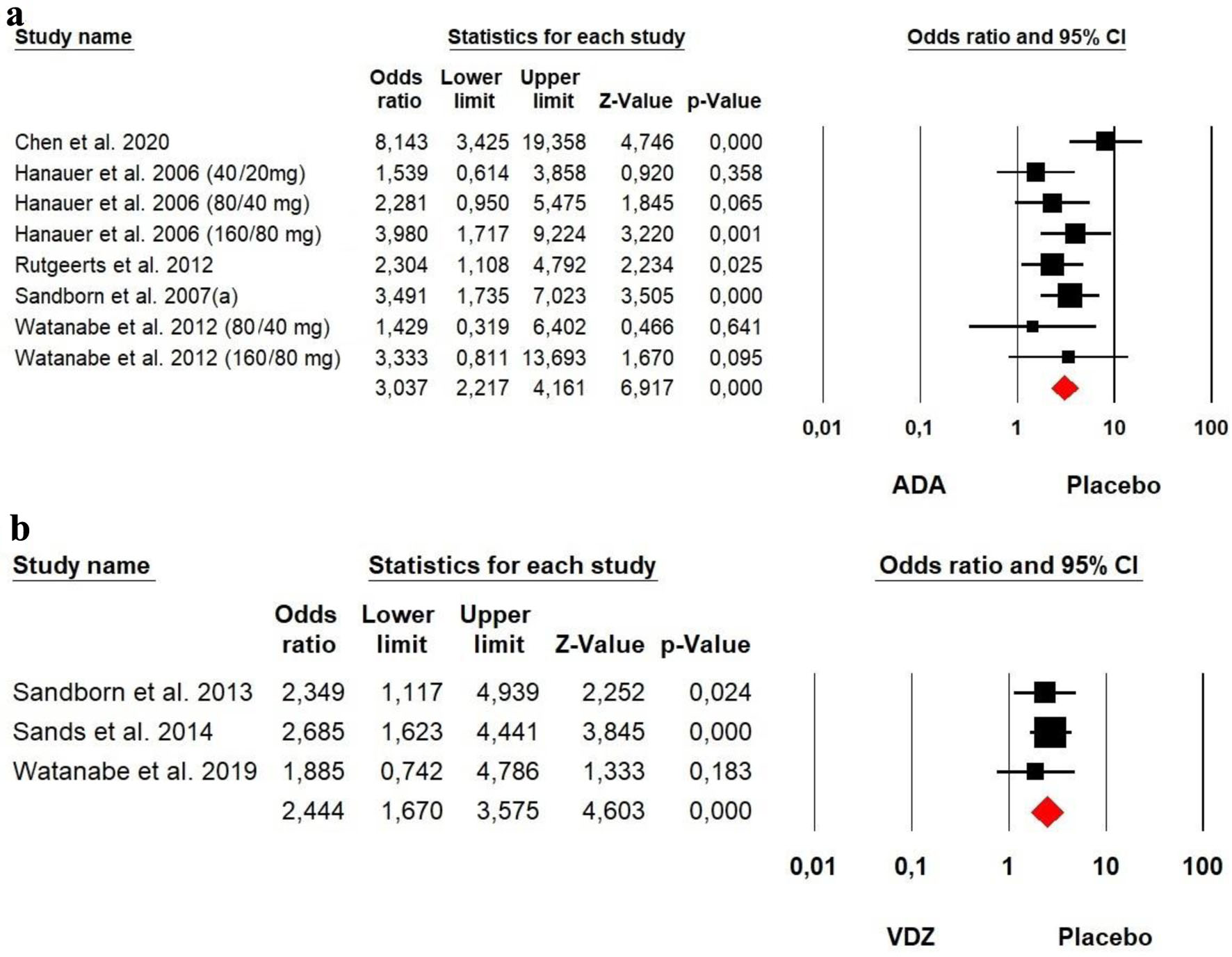
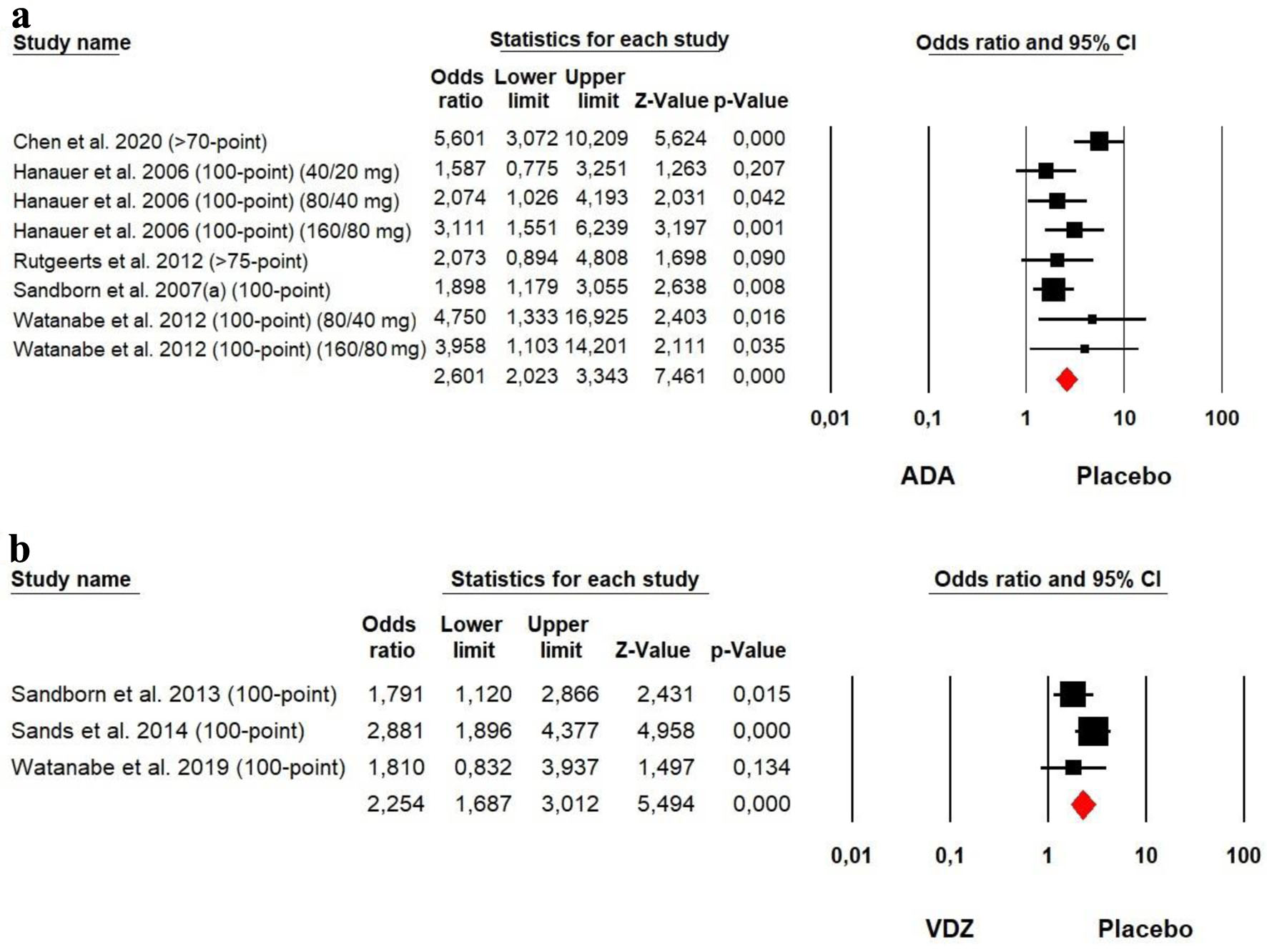
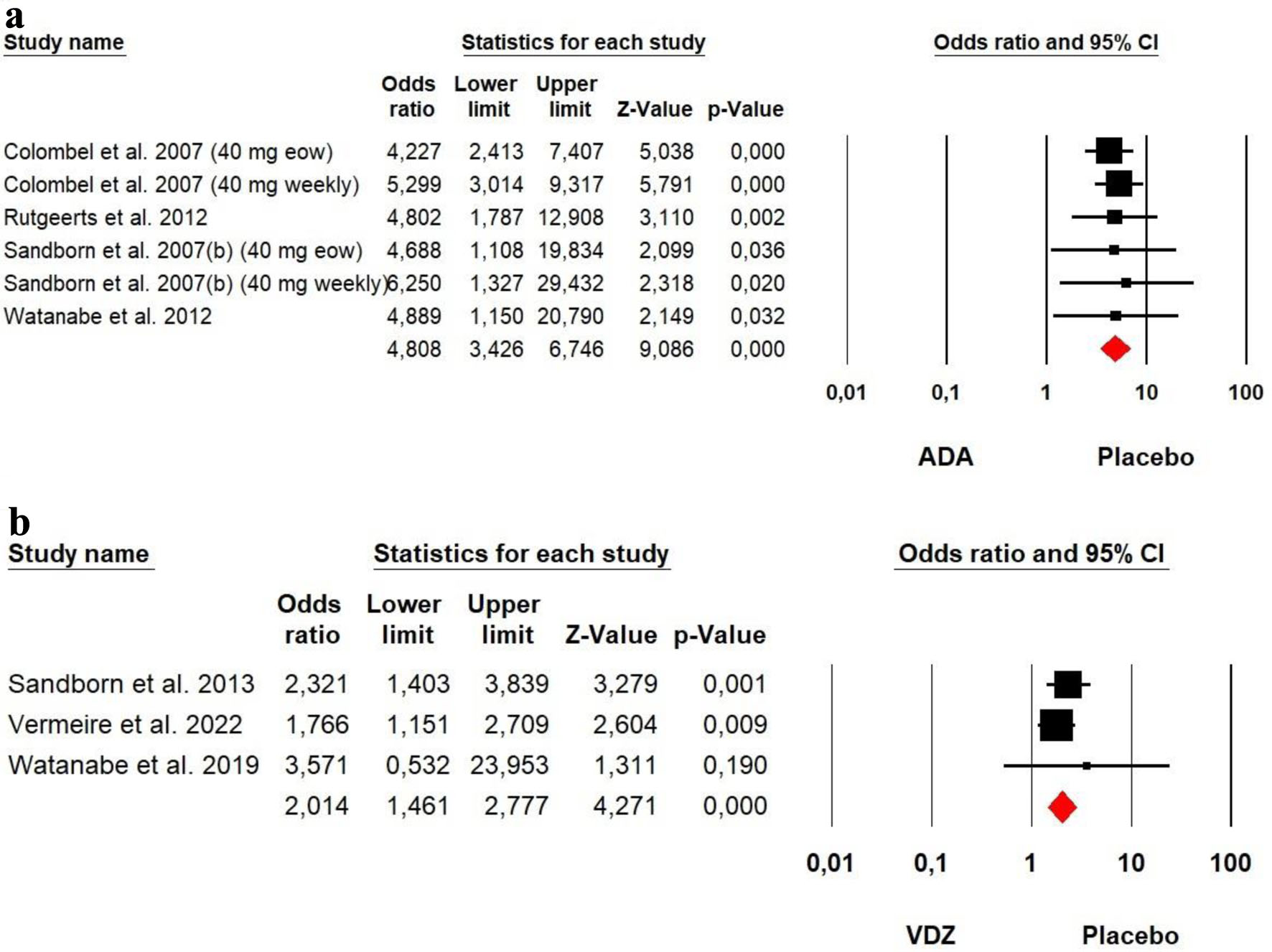
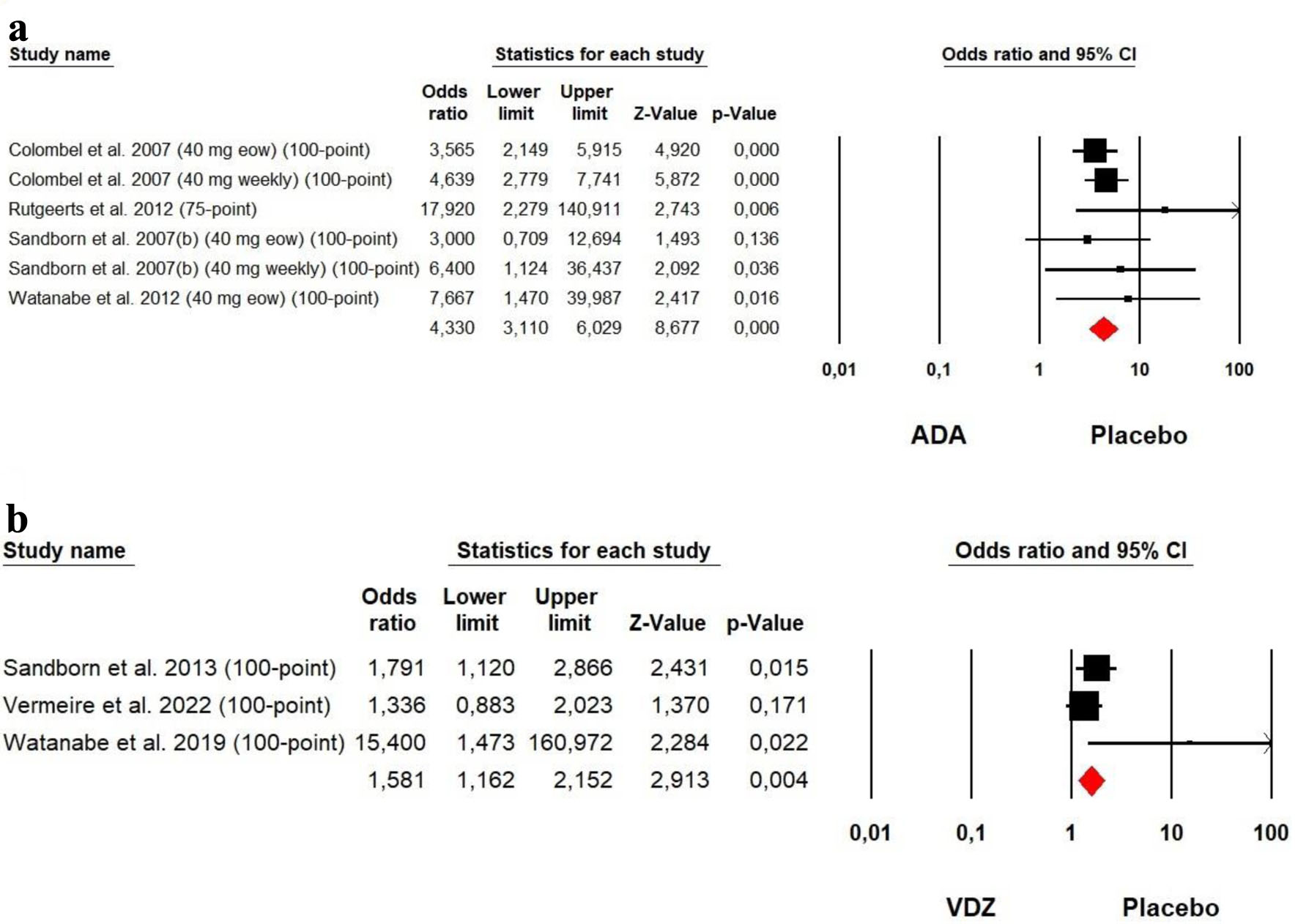
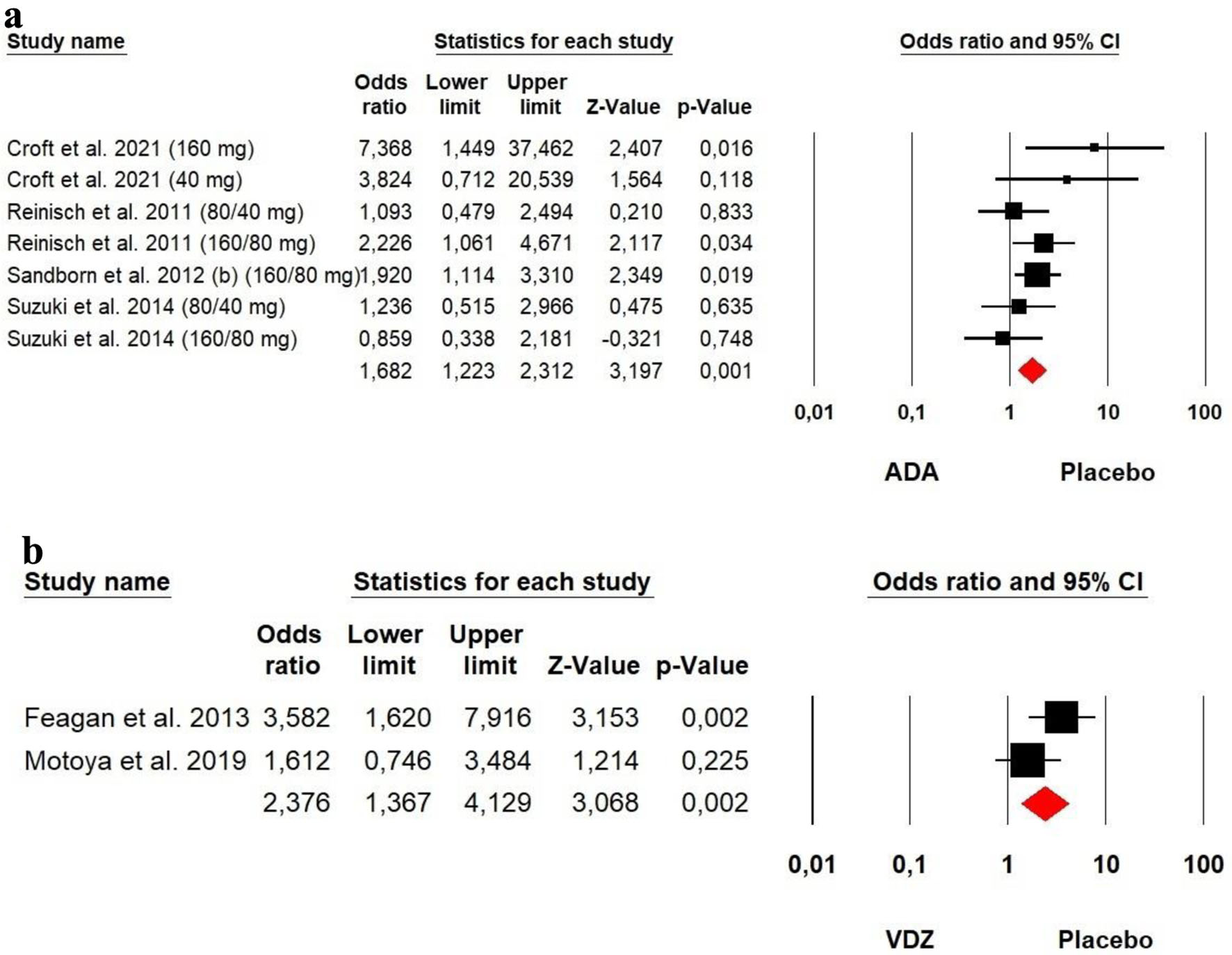
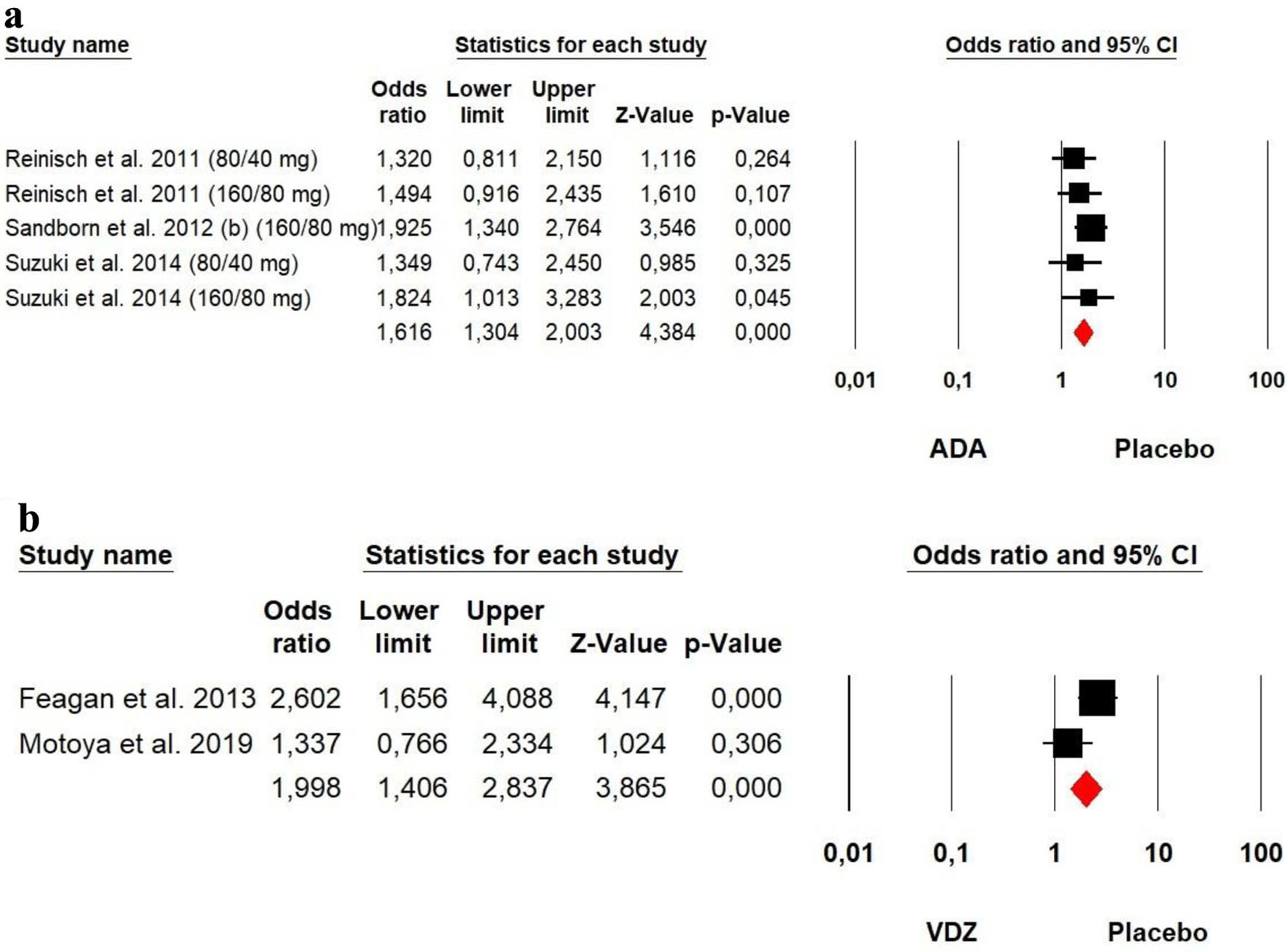
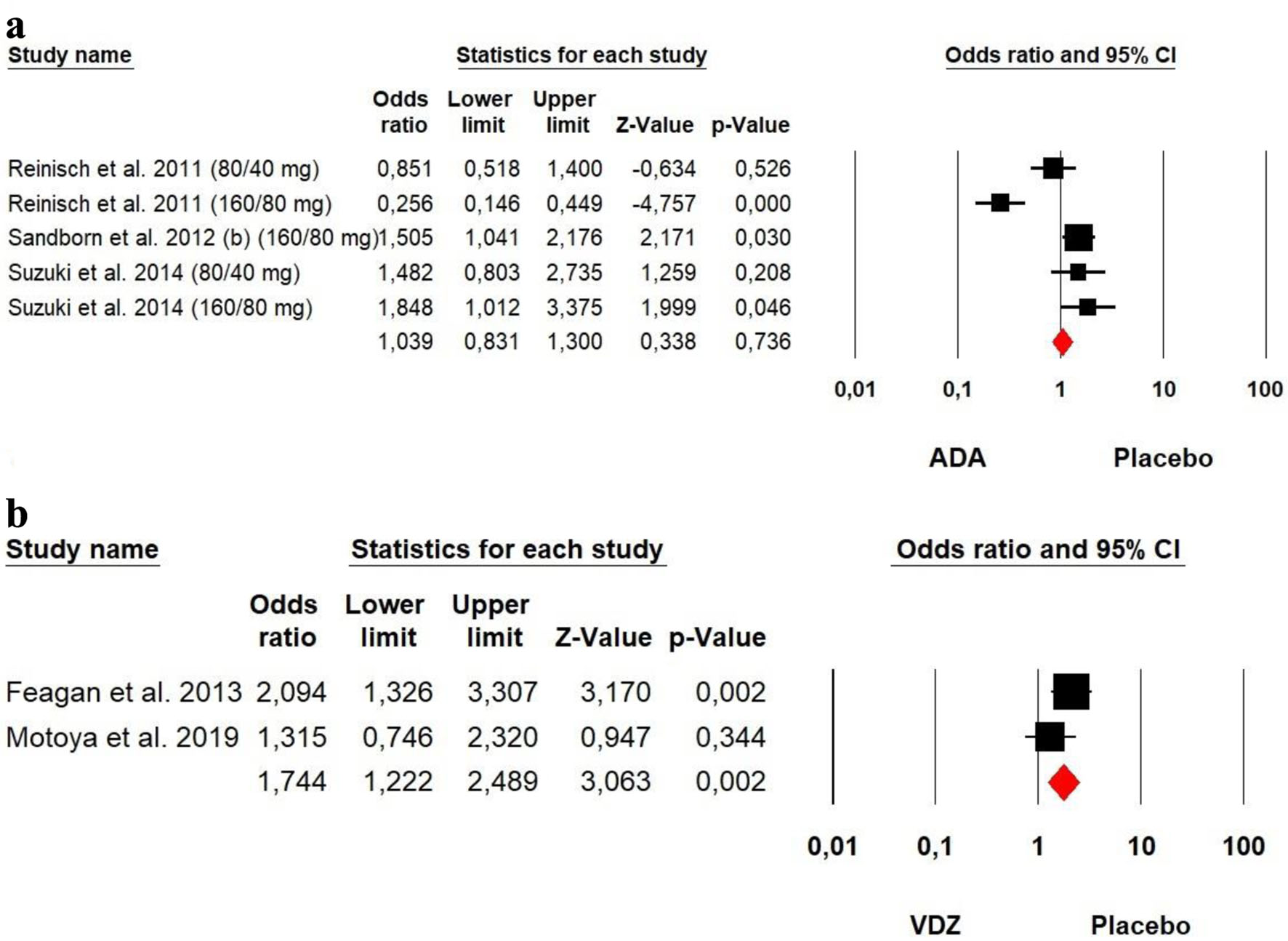
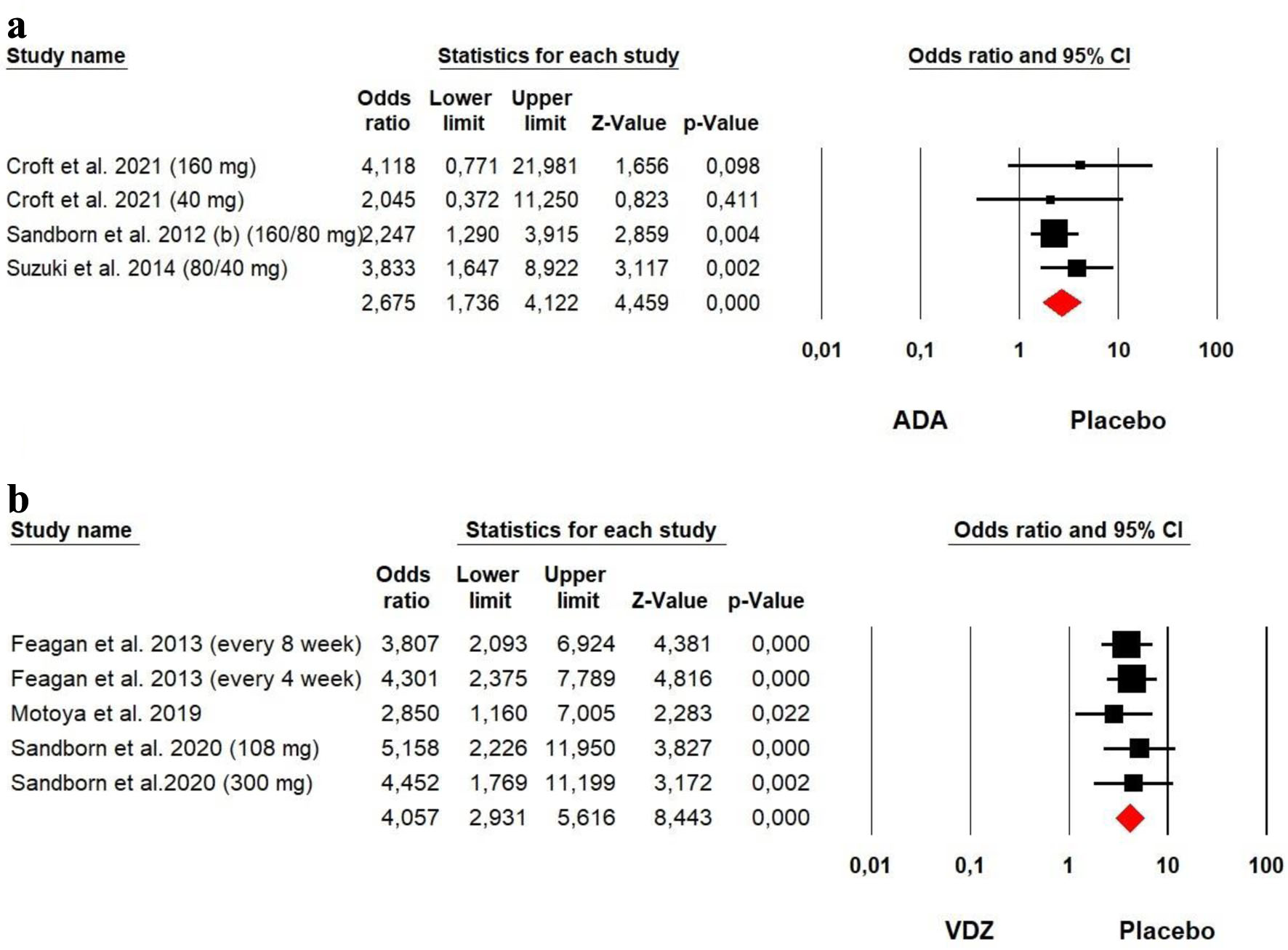
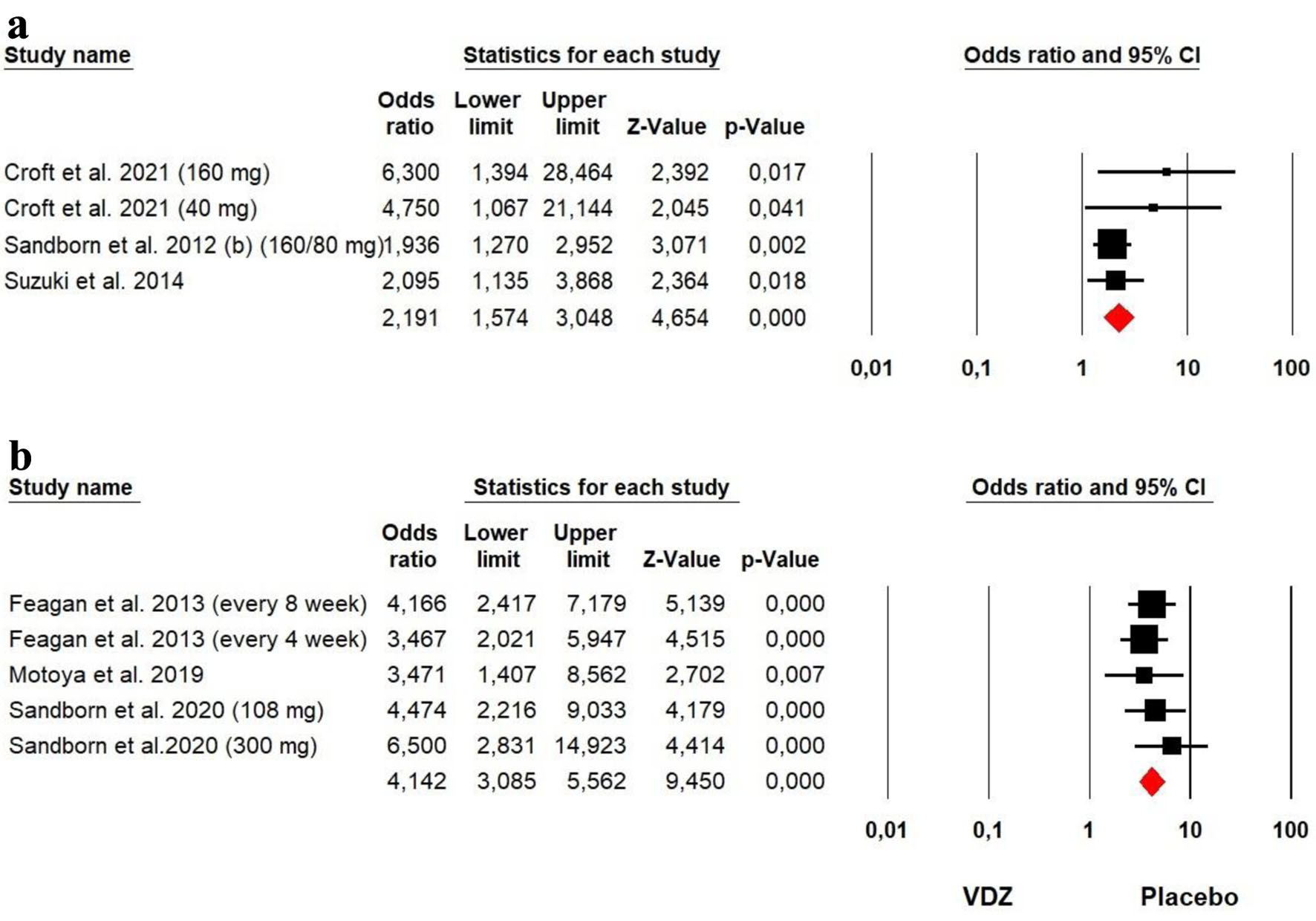
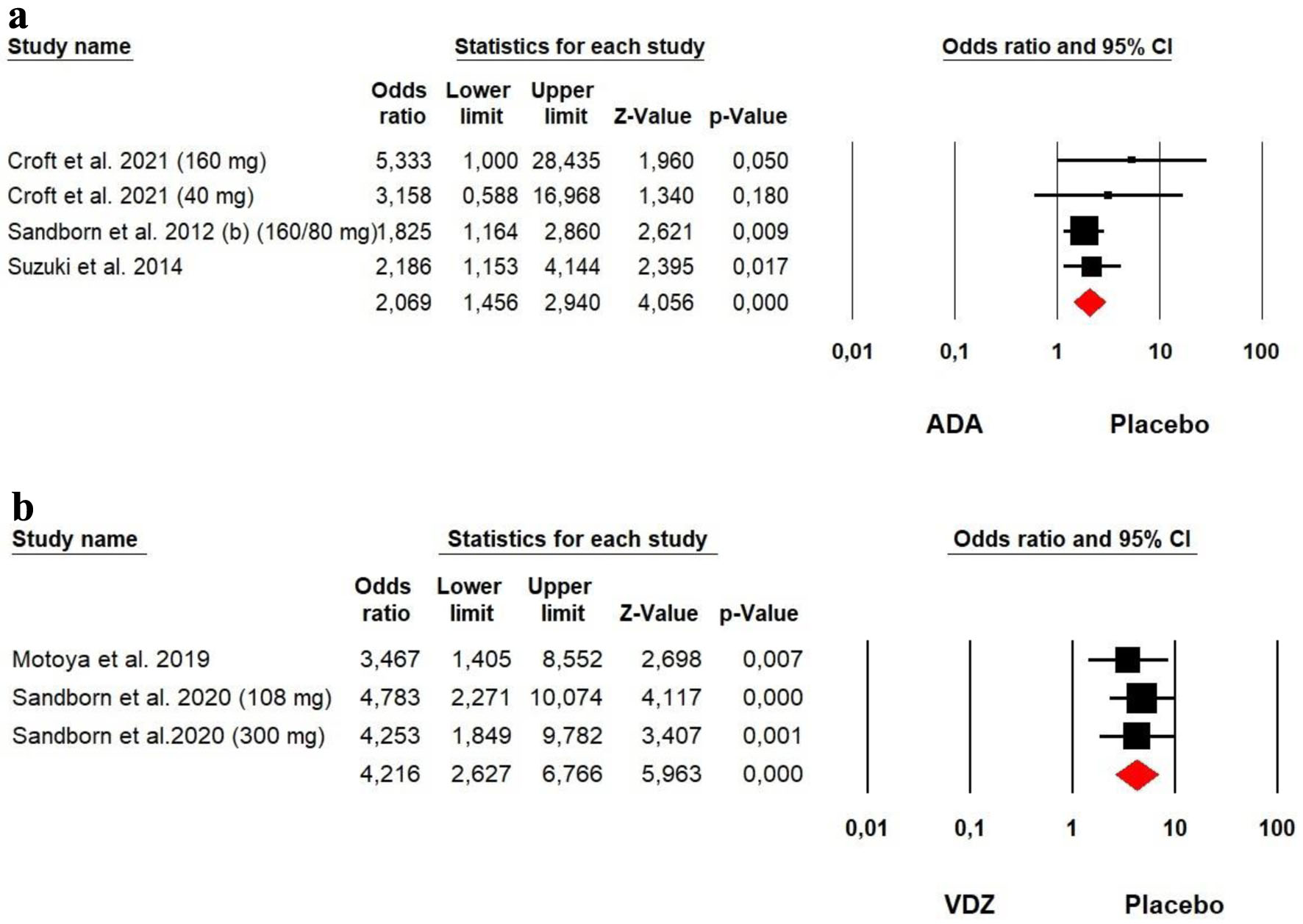
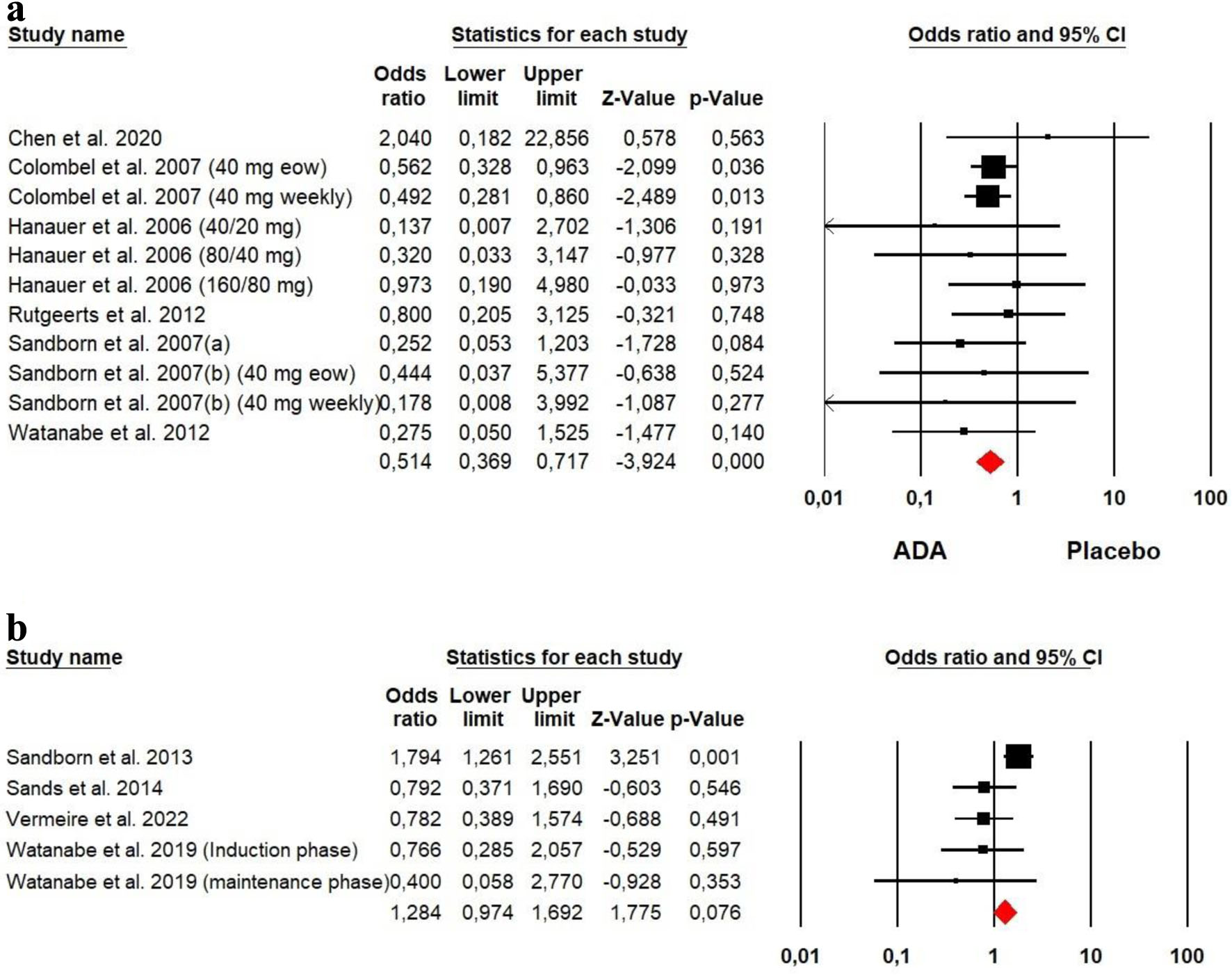
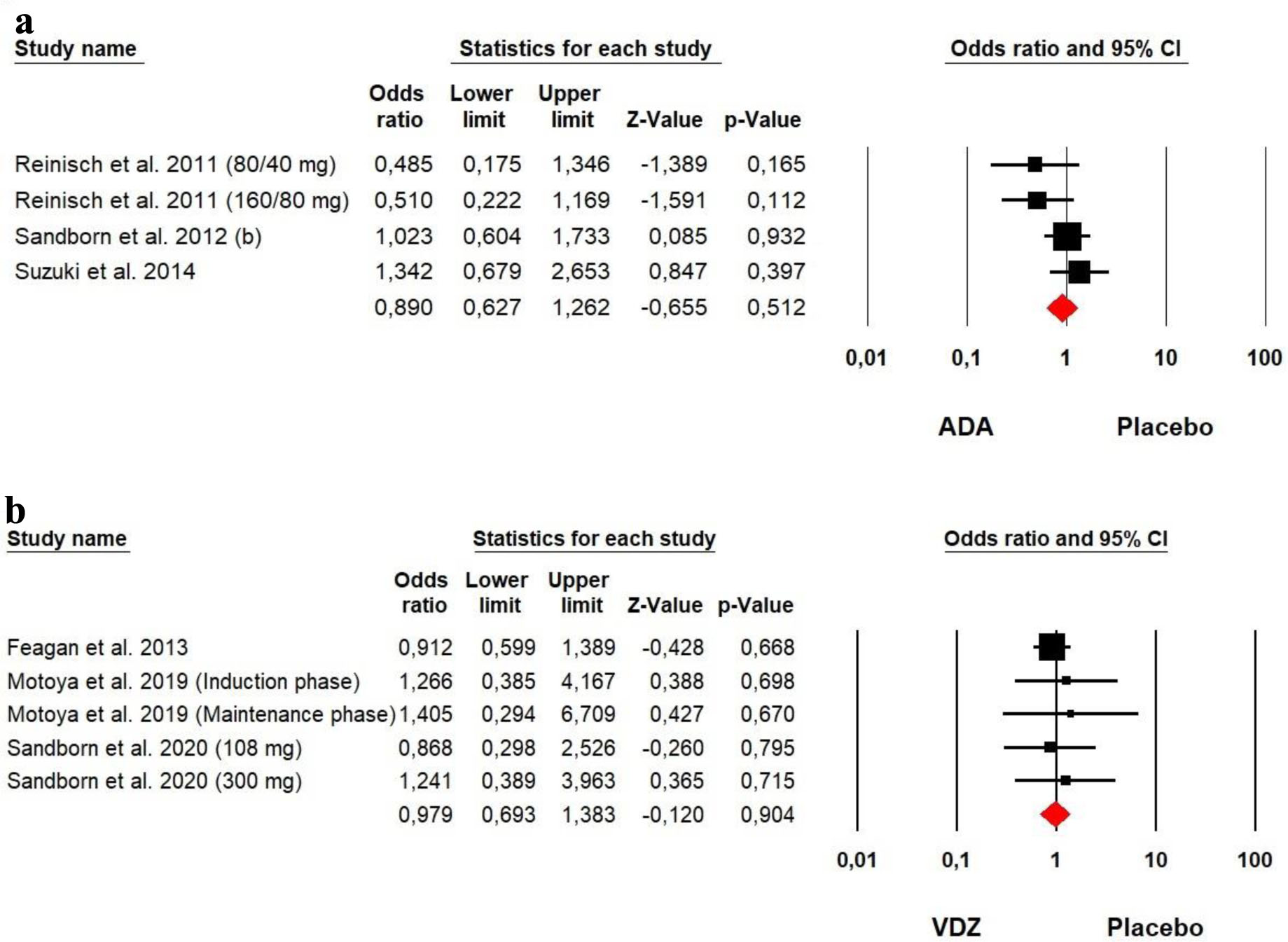
Tables
| Study | Location | Period | Design | Study phase | Name of trial | Sample size | Mean age, years | Male sex, % | Trial duration, weeks | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Chen et al, 2020 [12] | 15 sites in China | August 17, 2015 to December 15, 2017 | Multicenter, phase III randomized trial (The study comprised an 8-week double-blind (DB) period followed by an 18-week open-label (OL) period) | Induction | - | 205 | 32.9 | 68% | 26 | -Clinical remission at week 4. -Clinical response at week 4. -Adverse events |
| Colombel et al, 2007 [13] | 92 sites in Europe, the United States, and Canada | July 2003 to September 2005 | Multicenter, phase 3, randomized, double-blind, placebo-controlled trial | Maintenance | Crohn’s Trial of the Fully Human Antibody Adalimumab for Remission Maintenance (CHARM) | 854 | 37.1 | 11.9% | 56 | -Clinical response at weeks 26 and 56. -Clinical remission at weeks 26 and 56. -Adverse events |
| Hanauer et al, 2006 [14] | 55 centers in Europe, the United States, and Canada | July 24, 2002 to December 18, 2003 | Multicenter, randomized, double-blind, placebo-controlled trial | Induction | Clinical Assessment of Adalimumab Safety and Efficacy Studied as Induction Therapy in Crohn’s Disease (CLASSIC-I) | 299 | 38 | 46% | 4 | -Clinical remission at week 4. -Clinical response at week 4. -Adverse events |
| Rutgeerts et al, 2012 [15] | 19 sites in Europe, the United States, and Canada | August 2006 to September 2008 | Randomized, double-blind, placebo-controlled, maintenance/withdrawal study of adalimumab | Induction and Maintenance | Extend the safety and efficacy of adalimumab through endoscopic healing (EXTEND) | 129 | 37.1 | 37.2% | 52 | -Clinical remission at weeks 12 and 52. -Clinical response at weeks 12 and 52. -Mucosal healing at weeks 12 and 52. -Adverse events. |
| Sandborn et al, 2007a [16] | 52 sites in the United States, Canada, and Europe | November 2004 to December 2005 | Randomized, double-blind, placebo-controlled trial | Induction | - | 325 | 38 | 35% | 4 | -Clinical remission at week 4. -Clinical response at week 4. -Adverse events. |
| Sandborn et al, 2007b [17] | Multi-countries | August 28, 2002 to January 12, 2005 | Multi-center, randomized, double-blind, placebo-controlled trial | Maintenance | Clinical Assessment of Adalimumab Safety and Efficacy Studied as Induction Therapy in Crohn’s Disease (CLASSIC-II) | 55 | 36 | 40% | 56 | -Clinical remission at week 56. -Clinical response at week 56. -Adverse events. |
| Watanabe et al, 2012 [18] | Japan | January 2007 to December 2008 | Multicenter, randomized, double-blind, placebo-controlled trial | Induction and maintenance | - | 90 | 31.1 | 59.9% | 56 | -Clinical remission at week 4 and 56. -Clinical response at week 4 and 56. -Adverse events. |
| Sandborn et al, 2013 [19] | 285 medical centers in 39 countries | December 2008 to May 2012 | Phase 3, randomized, parallel-group, double-blind, placebo-controlled study | Induction and maintenance | GEMINI 2 | 1115 | 36.1 | 46.6% | 52 | -Clinical remission at week 6 and 52. -Clinical response at week 6 and 52. -Adverse events. |
| Sands et al, 2014 [20] | 107 sites in North America, Europe, Asia, Africa, and Australia | November 2010 to April 2012 | Phase 3, randomized, placebo-controlled, double-blind, multinational, multicenter trial | Induction | GEMINI 3 | 315 | 35.8 | 56.5% | 10 | -Clinical remission at week 6 and 10. -Clinical response at week 6 and 10. -Adverse events. |
| Vermeire et al, 2022 [21] | 169 sites in 30 countries | December 2015 to May 2019 | Randomized, double-blind, placebo-controlled, phase 3 trial | Maintenance | VISIBLE 2 | 409 | 37 | 53.2% | 52 | -Clinical remission at week 52. -Clinical response at week 52. -Adverse events. |
| Watanabe et al, 2020 [22] | 77 centers in Japan | January 28, 2014 to November 16, 2017 | A prospective, multicenter, randomized, double-blind, placebo-controlled phase 3 trial | Induction and maintenance | - | 157 | 34.6 | 64% | 60 | -Clinical remission at weeks 10 and 60. -Clinical response at weeks 10 and 60. -Adverse events. |
| Croft et al, 2021 [23] | 24 hospitals in 10 countries | January 1, 2014 to December 22, 2020 | A double-blind, placebo-controlled phase 3 trial | Induction and maintenance | ENVISION I | 93 | 14.1 | 45% | 52 | -Clinical remission at weeks 8 and 52. -Clinical response at week 52. -Mucosal healing at week 52. -Adverse events. |
| Reinisch et al, 2011 [24] | 94 centers in North America and Europe | August 2007 to February 2010 | Phase III, multicenter, randomized, double-blind, placebo-controlled trial | Induction | - | 390 | 37.5 | 62.3% | 8 | -Clinical remission at week 8. -Clinical response at week 8. -Mucosal healing at week 8. -Adverse events. |
| Sandborn et al, 2012 [25] | 103 centers in North America, Europe, Australia, New Zealand, and Israel | November 2006 and March 2010 | Phase 3, multicenter, randomized, double-blind, placebo-controlled trial | Induction and maintenance | - | 494 | 40.4 | 59.5% | 52 | -Clinical remission at weeks 8 and 52. -Clinical response at weeks 8 and 52. -Mucosal healing at weeks 8 and 52. -Adverse events. |
| Suzuki et al, 2014 [26] | Japan | February 2009 to May 2011 | Phase II/III, randomized, double-blind, placebo-controlled study | Induction and maintenance | - | 273 | 42.7 | 62.7% | 52 | -Clinical remission at weeks 8 and 52. -Clinical response at weeks 8 and 52. -Mucosal healing at weeks 8 and 52. -Adverse events. |
| Feagan et al, 2013 [27] | 211 medical centers in 34 countries from 2008 to 2012 | 2008 to 2012 | Phase 3, randomized, double-blind, placebo-controlled study | Induction and maintenance | - | 895 | 40.3 | 58.7% | 52 | -Clinical remission at weeks 6 and 52. -Clinical response at week 6. -Mucosal healing at weeks 6 and 52. -Adverse events. |
| Motoya et al, 2019 [28] | 86 sites in Japan | February 2014 to November 2015 | Phase 3, randomized, double-blind, placebo-controlled study | Induction and maintenance | - | 292 | 42.9 | 61.3% | 60 | -Clinical remission at weeks 10 and 60. -Clinical response at weeks 10 and 60. -Mucosal healing at weeks 10 and 60. |
| Sandborn et al, 2020 [29] | 141 sites in 29 countries | December 18, 2015 to August 21, 2018 | Phase 3, randomized, placebo-controlled, double-blind, double-dummy trial | Maintenance | VISIBLE 1 | 216 | 39.7 | 59.8% | 52 | -Clinical response at week 52. -Clinical remission at week 52. -Mucosal healing at week 52. -Adverse events. |
| Study | The Jadad scores | Total score | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| 1: Was the study carried out using randomization? 2: Did the study provide a clear and suitable description of the randomization process? 3: Was the study characterized as double-blind? 4: Was the blinding procedure described meticulously and aptly? 5: Were details regarding withdrawals and dropouts included? | ||||||
| Chen et al, 2020 [12] | 1 | 0 | 1 | 1 | 1 | 4 |
| Colombel et al, 2007 [13] | 1 | 0 | 1 | 1 | 1 | 4 |
| Hanauer et al, 2006 [14] | 1 | 0 | 1 | 1 | 1 | 4 |
| Rutgeerts et al, 2012 [15] | 1 | 0 | 1 | 1 | 0 | 3 |
| Sandborn et al, 2007a [16] | 1 | 0 | 1 | 1 | 1 | 4 |
| Sandborn et al, 2007b [17] | 1 | 0 | 1 | 1 | 1 | 4 |
| Watanabe et al, 2012 [18] | 1 | 0 | 1 | 1 | 1 | 4 |
| Sandborn et al, 2013 [19] | 1 | 0 | 1 | 1 | 0 | 3 |
| Sands et al, 2014 [20] | 1 | 0 | 1 | 1 | 1 | 4 |
| Vermeire et al, 2022 [21] | 1 | 0 | 1 | 1 | 0 | 3 |
| Watanabe et al, 2020 [22] | 1 | 0 | 1 | 1 | 0 | 3 |
| Croft et al, 2021 [23] | 1 | 0 | 1 | 1 | 1 | 4 |
| Reinisch et al, 2011 [24] | 1 | 0 | 1 | 1 | 0 | 3 |
| Sandborn et al, 2012 [25] | 1 | 0 | 1 | 1 | 0 | 3 |
| Suzuki et al, 2014 [26] | 1 | 0 | 1 | 1 | 1 | 4 |
| Feagan et al, 2013 [27] | 1 | 0 | 1 | 1 | 0 | 3 |
| Motoya et al, 2019 [28] | 1 | 0 | 1 | 1 | 0 | 3 |
| Sandborn et al, 2020 [29] | 1 | 0 | 1 | 1 | 1 | 4 |