| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Original Article
Volume 16, Number 4, August 2023, pages 226-233
Effects of Helicobacter pylori Infection on the Prognosis of Chronic Atrophic Gastritis by Inducing the Macrophage Polarization
Chun Na Zhaoa, b, Li Li Xiaoa, b, Ying Zhanga, c
aDepartment of Gastroenterology, Daqing Oilfield General Hospital, Daqing City, Heilongjiang Province, 163000, China
bThese authors contributed equally to this manuscript.
cCorresponding Author: Ying Zhang, Department of Gastroenterology, Daqing Oilfield General Hospital, Daqing City, Heilongjiang Province, 163000, China
Manuscript submitted May 3, 2023, accepted June 22, 2023, published online July 12, 2023
Short title: Effect of H. pylori Infection on CAG
doi: https://doi.org/10.14740/gr1636
| Abstract | ▴Top |
Background: Recently, the effects of Helicobacter pylori (H. pylori) infection on the prognosis of chronic atrophic gastritis (CAG) are still unclear. The aim of our study was to discuss the role of H. pylori infection on the prognosis of CAG by inducing the M1/M2 macrophage polarization.
Methods: A total of 180 subjects as control (group 1), CAG patients without H. pylori infection (group 2) and H. pylori-associated CAG patients (group 3) were respectively recruited for this cross-sectional investigation in Daqing Oilfield General Hospital from May 2019 to July 2020. Their serum samples were collected to determine the concentrations of pro-inflammatory and anti-inflammatory cytokines. Meanwhile, the gastric mucosa was excised to determine the related gene expressions on the M1/M2 macrophage polarization. Then the prognosis of CAG was evaluated according to the status of clinical manifestations and endoscopic examination after the follow-up.
Results: Notably, it was proved that compared with the control group, the expressions and concentrations of pro-inflammatory cytokines (M1 macrophage: inducible nitric oxide synthase (iNOS), tumor necrosis factor-alpha (TNF-α), interferon-gamma (IFN-γ), and interleukin-6 (IL-6)) were significantly higher, while the anti-inflammatory cytokines (M2 macrophage: arginase-1 (Arg-1), IL-4 and IL-10) were apparently reduced in the group 2 and group 3 (P < 0.05). Moreover, more days were needed for the prognosis of CAG in group 3 than those in group 2, which was accompanied by higher expressions of pro-inflammatory and lower anti-inflammatory cytokines at the baseline (P < 0.05). Furthermore, negative correlations were shown between the concentrations of iNOS, TNF-α, IFN-γ and IL-6, and the prognosis of CAG (P < 0.05), while positive correlations were observed between the contents of IL-4 and IL-10, and prognosis of CAG (P < 0.05).
Conclusion: These above results indicated that H. pylori infection-induced disorders of M1/M2 macrophage polarization could affect the prognosis of CAG.
Keywords: Chronic atrophic gastritis; Helicobacter pylori; Macrophage polarization; Pro-inflammatory; Anti-inflammatory
| Introduction | ▴Top |
Chronic atrophic gastritis (CAG), as a prevailing disease in the digestive outpatients, is characterized by the chronic inflammatory processes of gastric mucosa, altered mucosal atrophy and intestinal metaplasia [1], so it is ultimately served as the main stage on the precancerous lesion of irreversible tumorigenesis [2]. However, its specific mechanism is still unclear, so there was an urgent need to discuss the more effective intervention and prevention strategies.
Up to now, a lot of declared elements were proved to affect the prognosis of CAG, in which the Helicobacter pylori (H. pylori) infection was recognized as an important risk factor [3, 4]. As a significant human gram-negative and microaerophilic bacterium, the colonization of H. pylori in the stomach mucosa could induce both innate and adaptive immune responses in the gastric mucosa to aggravate the symptoms of CAG [5, 6], in which the macrophages represent a dynamic subset of innate immune cells and coordinate the immune responses to the infection of H. pylori timely [7, 8]. As we all know, macrophages make up the majority of leukocytes and display a high plasticity [9]. There were two major characterized phenotypes of macrophage polarization according to the different micro-environmental and cytokines stimulation, including the classically activated type 1 (M1) and alternatively activated type 2 (M2) macrophages as the extremes of the continuum of functional states [10]. In this condition, the infiltration of M1 macrophages was characterized by the production of pro-inflammatory cytokines like interleukin-1β (IL-1β), interleukin-6 (IL-6), interferon-γ (IFN-γ) and tumor necrosis factor-alpha (TNF-α), while the expression of inducible nitric oxide synthase (iNOS), the enzyme responsible for NO production by M1 macrophages, is specific to murine macrophages. Meanwhile, M2 macrophages polarization can be induced by the different stimulation, such as interleukin-4 (IL-4) and/or interleukin-13 (IL-13), immune complexes and Toll-like receptor (arginase-1 (Arg-1)), IL-1 receptor ligands or interleukin-10 (IL-10) to play their pro-angiogenic and pro-tumorigenic roles [9-11].
Obviously, the balance relationships exist between M1 and M2 macrophages in healthy population, so the polarization status of anti-inflammatory M2 macrophages will switch to a more pro-inflammatory M1 state to promote the occurrence of inflammation under the status of infection [12, 13]. As described in the references [13], the H. pylori infection could play an important role in the occurrence of many gastric diseases, just as CAG. Nevertheless, the potential mechanisms by which H. pylori affects the prognosis of CAG are not yet completely clarified. Therefore, in this study, we aimed to discuss the role of H. pylori infection in the prognosis of CAG and seek possible mechanism from the M1/M2 macrophage polarization using 180 subjects in the Department of Gastroenterology in Daqing Oilfield General Hospital.
| Materials and Methods | ▴Top |
Study design and population
This cross-sectional survey was conducted in the Department of Gastroenterology in Daqing Oilfield General Hospital from May 2019 to July 2020. All consecutive subjects were aged over 18 and below 65 years old in the outpatient department of digestive medicine. Sixty healthy subjects without CAG were chosen as the controls (group 1), who were accompanied with the symptoms of stomach discomfort to some extent and they were voluntarily participated in this study by the gastroscopy and pathological biopsies as a record. While the CAG patients were with upper gastrointestinal (GI) symptoms, including epigastric pain, abdominal bloating, postprandial fullness, early satiety, belching, regurgitation, heartburn, appetite loss and nausea, who were obviously diagnosed by the clinicopathological evaluation after the gastroscopy samplings [14]. The pathological assessment of gastric mucosa in all groups was calculated and the “mean” atrophy severity scores were added according to the Operative Link on Gastritis Assessment (OLGA) (0: no atrophy of gastric mucosa; 1: mild atrophy of gastric mucosa; 2: moderate atrophy of gastric mucosa; 3: severe atrophy of gastric mucosa). Then all CAG patients were divided into two groups according to the results of 13C urea breath test (13C-UBT) as the group 2 (n = 60, CAG patients without H. pylori infection) and group 3 (n = 60, H. pylori-associated CAG patients) [15, 16].
All the involving subjects underwent first diagnostic upper endoscopy according to the pathological results, in which the endoscopic diagnosis of CAG was consistent with the classification and grading criteria by Chinese Society of Digestive Endoscopy [14]. The exclusion criteria included the usage of some drugs (proton pump inhibitors (PPIs), non-steroidal anti-inflammatory drugs (NSAIDs), antiplatelet agents and anticoagulants), history of GI malignancy, weight loss, hematemesis, melena, rectal bleeding, vomiting, dysphagia and anemia, severe cardiovascular, renal and pulmonary co-morbidities, mental illness or pregnancy/lactation women. What is more, the subjects with reflux esophagitis, peptic ulcer, polyp, and cancer at endoscopy were also excluded.
The ethical approval was approved by Medical Ethics Committee of Daqing Oilfield General Hospital in China (2018-08-01). All experiments were carried out in accordance with relevant guidelines and regulations “Declaration of Helsinki (revised October 2013)”.
Interview
According to the inclusion and exclusion criteria, all subjects were interviewed by the questionnaires about their medication, current smoking, previous fractures, dietary intake as well as the basic data, such as the age, height, weight, sex, etc.
Determination of serum pro-inflammatory and anti-inflammatory cytokines
Two milliliter of venous peripheral blood was drew and assigned to the vacuum blood collection tubes (EDTA, Beijing, China) before the clinical treatment to determine the serum pro-inflammatory (TNF-α, IFN-γ and IL-6) and anti-inflammatory cytokines (IL-4 and IL-10) by the enzyme-linked immunosorbent assay (ELISA) kits following the instructions (Biohit Health Care).
RT-PCR of genes on M1 and M2 macrophage polarization
Total RNA in the gastric mucosa was extracted using the TRIzol Reagent (cat. no. 15596-206, Invitrogen, Carlsbad, CA, USA), and cDNA was reverse transcribed by SuperScript™ III First-Strand Synthesis System for RT-PCR (cat. no. 18080-051, Invitrogen). Then the expression levels of genes on the pro-inflammatory (iNOS, TNF-α, IFN-γ, IL-6 and IL-1β) and those coding for anti-inflammatory cytokines (Arg-1, IL-4 and IL-10) were determined by RT-PCR (CFX-96, Bio-Rad, USA), while the β-actin gene was used as internal control. All assays were performed in triplicates and normalized with the internal standard mRNA levels using the 2-▲▲CT method, in which the oligonucleotide primers were designed using Primer-BLAST and listed in Table 1.
 Click to view | Table 1. Primer Sequences of Related Genes for RT-PCR |
Follow-up: the prognosis of CAG
After 2 weeks’ conventional therapy, the CAG patients in group 2 and group 3 were followed up and their prognosis was evaluated according to the status of clinical manifestations and endoscopic results [3].
Statistical analysis
The statistical analyses were conducted using SPSS 21.0. All values were expressed as mean ± standard error (SE) among the three groups, in which Shapiro-Wilk and Percent-Percent plot (P-P Figure) were chosen to determine the normality of data. Then one-way analysis of variance (ANOVA) was performed to compare the means of indexes among the different groups with normally distributed data, then the Student-Newman-Keuls (SNK) test was used to determine where the differences existed between each two groups. Whereas the data with non-normal distribution were assessed using Mann-Whitney U-test. The value of P < 0.05 was considered to be statistically significant.
| Results | ▴Top |
Characteristic of the general information
A total of 180 subjects were recruited in this study, including 60 subjects in the control group (group 1), 60 CAG patients without H. pylori infection (group 2) and 60 H. pylori-associated CAG patients (group 3). As shown in Table 2, there were no significant differences on the distribution of gender, age, height, weight, body mass index (BMI), smoking and alcohol consumption among these three groups (P > 0.05). Meanwhile, the types of CAG and atrophy severity scores were not significantly different between groups 2 and 3 (P > 0.05). However, the prognosis of CAG was better in group 2 (n = 57, 95%) than in group 3 (n = 39, 65%) after the routine treatment (P < 0.05).
 Click to view | Table 2. Characteristics of the Subjects in Each Group |
mRNA expressions on the M1/M2 macrophage polarization in the gastric mucosa
The mRNA expressions on the M1 macrophage polarization (iNOS, TNF-α, IFN-γ and IL-6) were significantly higher in groups 2 and 3 than those in group 1 (Fig. 1a, P < 0.05), whereas the expressions on the M2 macrophage polarization (Arg-1, IL-4 and IL-10) were apparently reduced (Fig. 1b, P < 0.05). Furthermore, among the CAG patients in groups 2 and 3, the mRNA levels of iNOS, IL-1β, TNF-α, INF-γ and IL-6 were much higher and those of IL-4 and IL-10 were lower in group 3 than those in group 2 (P < 0.05).
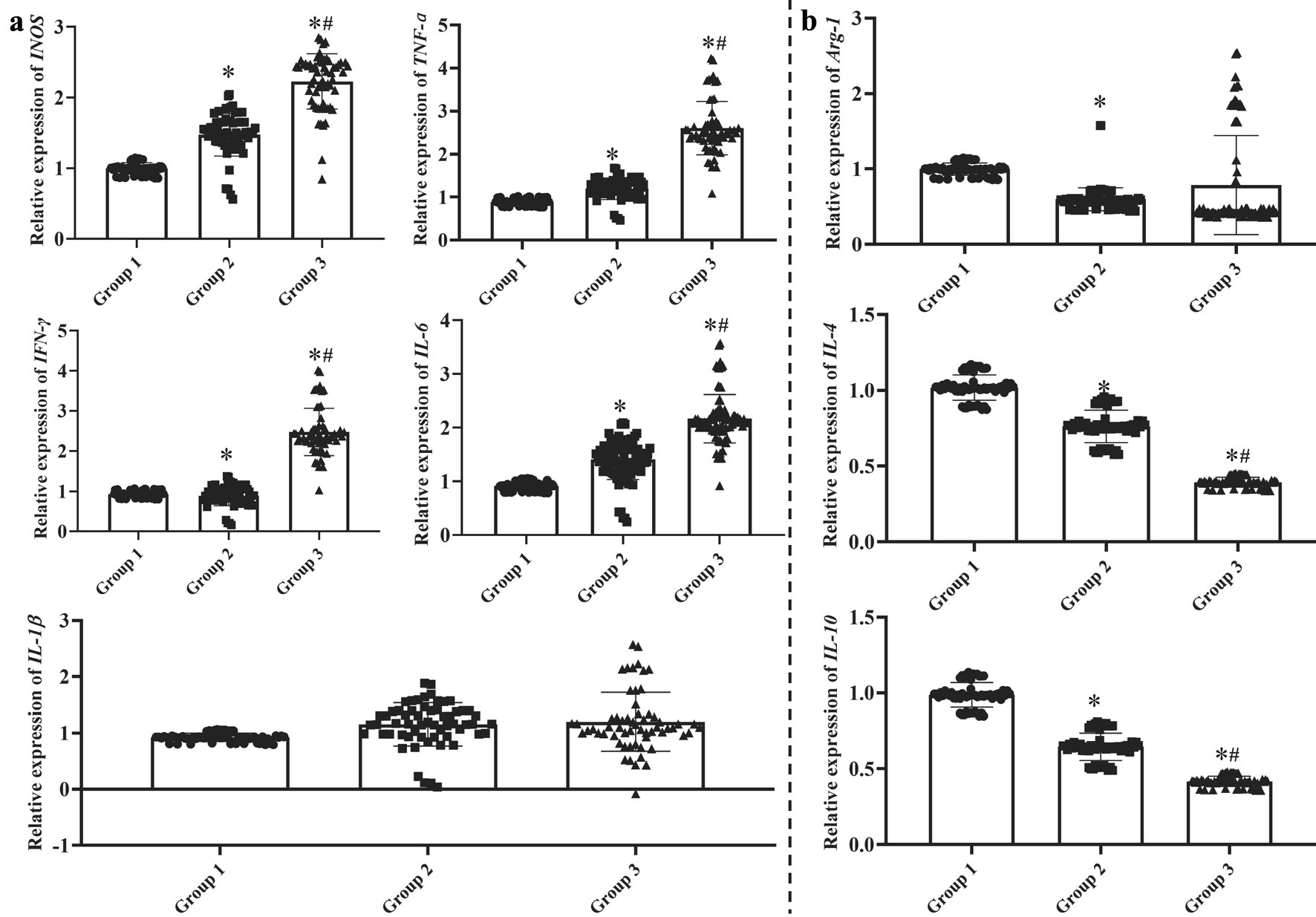 Click for large image | Figure 1. The mRNA expression on the M1 macrophage (a) and M2 macrophage (b) polarization in the gastric mucosa. Group 1: control group with health subjects; group 2: chronic atrophic gastritis patients without H. pylori infection; group 3: H. pylori-associated chronic atrophic gastritis patients. One-way analysis of variance (ANOVA) was performed to compare the means of these indexes, then the Student-Newman-Keuls (SNK) test was used to determine where the differences were existed between each two groups. *P < 0.05 compared with the group 1. #P < 0.05 compared with the group 2. |
Concentrations of serum pro-inflammatory and anti-inflammatory cytokines
As shown in Figure 2, the concentrations of TNF-α, IFN-γ and IL-6 were higher in groups 2 and 3 than those in group 1 (Fig. 2a, P < 0.05), whereas the contents of IL-4 and IL-10 were apparently decreased (Fig. 2b, P < 0.05). Furthermore, among the CAG patients, the contents of TNF-α, INF-γ and IL-6 were much higher and those of IL-4 and IL-10 were lower in group 3 than those in group 2 (P < 0.05).
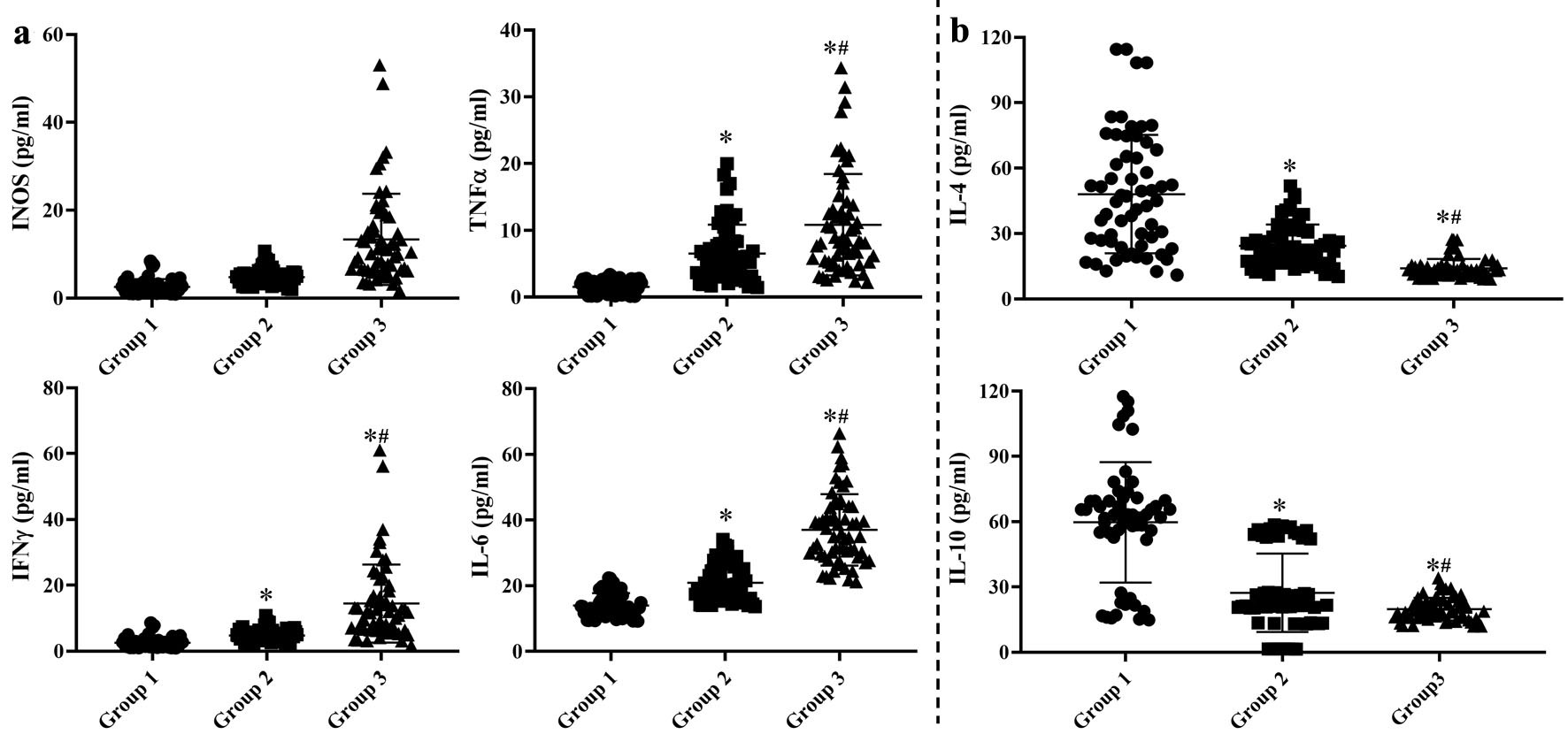 Click for large image | Figure 2. Concentrations of serum pro-inflammatory (a) and anti-inflammatory (b) cytokines. Group 1: control group with health subjects; group 2: chronic atrophic gastritis patients without H. pylori infection; group 3: H. pylori-associated chronic atrophic gastritis patients. One-way analysis of variance (ANOVA) was performed to compare the means of these indexes, then the Student-Newman-Keuls (SNK) test was used to determine where the differences were existed between each two groups. *P < 0.05 compared with the group 1. #P < 0.05 compared with the group 2. |
Correlations between the mRNA expressions in the gastric mucosa with their serum concentrations and the prognosis of CAG
The above results for the differently significant expressions of M1 (iNOS, TNF-α, IFN-γ and IL-6) and M2 macrophage polarization (IL-4 and IL-10) in the gastric mucosa, and their correlations with their concentrations of serum cytokines and the prognosis of CAG were further analyzed using the multiple linear regression models. As shown in Figure 3, there were positive correlations between the expressions of TNF-α, IFN-γ, IL-6, IL-4 and IL-10, and their serum concentrations (P < 0.05). Meanwhile, negative correlations were shown between the expressions of iNOS, TNF-α, IFN-γ and IL-6, and the prognosis of CAG (P < 0.05), while positive correlations were observed between the expressions of IL-4 and IL-10, and prognosis of CAG (P < 0.05).
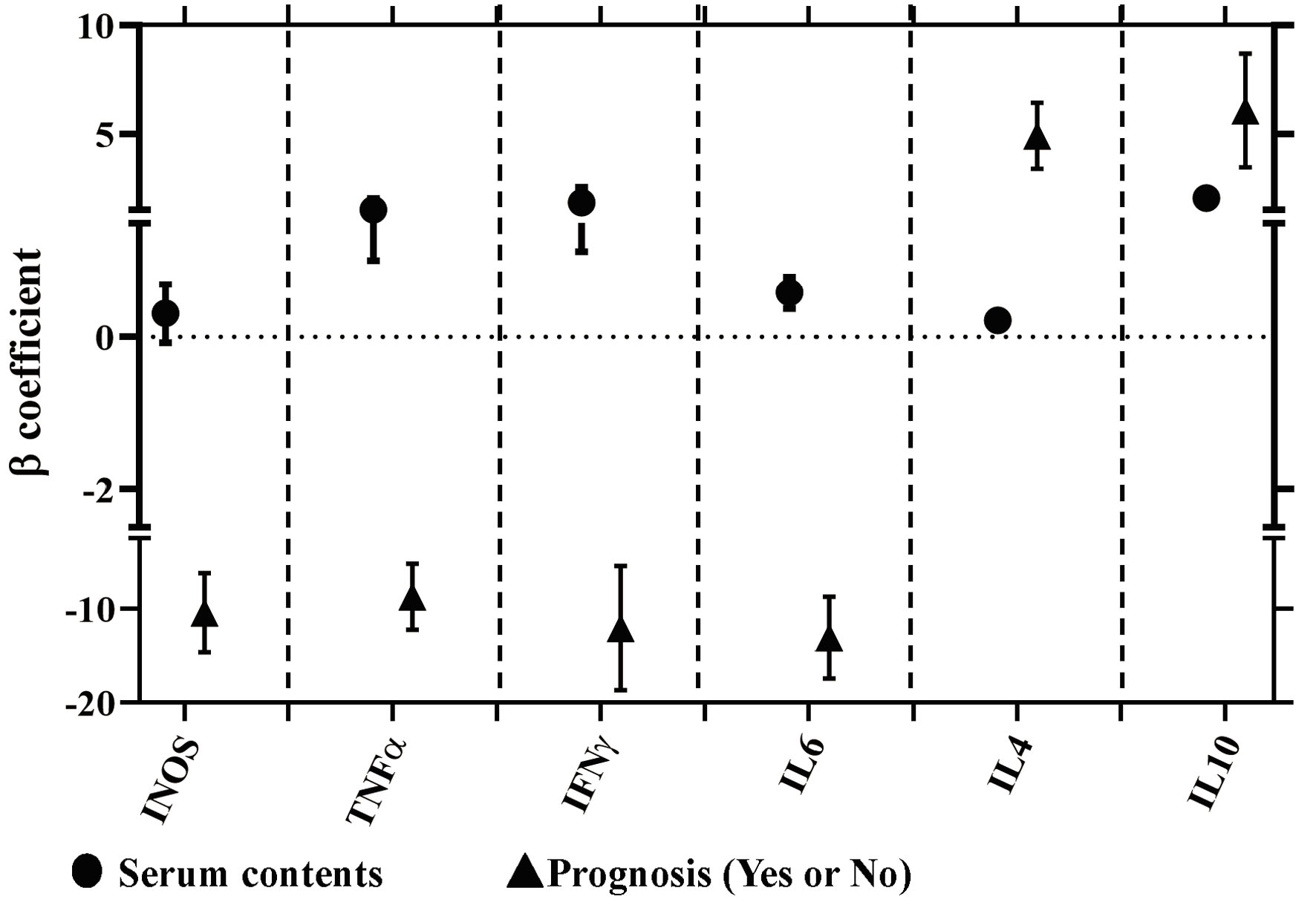 Click for large image | Figure 3. Correlations between the mRNA expressions in the gastric mucosa with their concentrations of serum cytokines and the prognosis of CAG using the multiple linear regression models. Circle represents the correlations between the concentrations of serum cytokines and related mRNA expression in the gastric mucosa. Triangle represents the correlations between the expressions of mRNA expressions in the gastric mucosa and the prognosis of CAG. Meanwhile, the positive, negative and no correlations were respectively represented by the β coefficient with more than zero (positive correlation), less than zero (negative correlation) and cross zero (no correlation). |
| Discussion | ▴Top |
Recently, several epidemiological and experimental studies have indicated that gastric cancer is the third leading cause of death in all types of malignancies behind lung and colorectal cancer [17], in which one of major risk factors of gastric cancer is H. pylori infection [18]. H. pylori could induce the chronic inflammation, autophagy, oxidative stress and subsequent carcinogenesis, in which a variety of factors were included: the H. pylori strain-specific virulence factors (especially the cytotoxin-associated gene A, vacuolating cytotoxin A and neutrophil activating protein), host and environmental factors such as diet as well as alternations in the stem cell populations and microbiome to play a fundamental role in causing DNA damage and promoting the invasion and migration of gastric cancer cells, with both processes often supporting each other. Then H. pylori infection results in a reduced ability of the infected cells to repair DNA damage to increase genetic instability and progressively lead to the accumulation of mutations, which could activate the oncogenes and deactivate tumor suppressor genes to increase the risk of developing gastric cancer. Besides, the metabolites of H. pylori, including the related enzymes and toxins, can directly activate cell signaling pathways such as phosphatidylinositol 3-kinases (PI3K)/Akt, AKT-nuclear factor-kappaB (NF-κB), Janus kinase/signal transducers and activators of transcription (JAK/STAT) and Ras, Raf, and extracellular signal-regulated kinase (ERK) signaling that control cell proliferation. Uncontrolled proliferation can lead to the occurrence of gastric cancer [19, 20]. So CAG induced by H. pylori could lead to the atrophic mucosa to increase the risk of gastric cancer. Moreover, it has been revealed that H. pylori eradication therapy is effective for the reduction of CAG, even the gastric cancer. A previous study demonstrated that CAG was associated with a variety of chronic inflammation, immune responses, etc. [21]. Furthermore, H. pylori infection could aggravate the occurrence of CAG [22]. However, it still remains uncertain whether there were some correlations between H. pylori and prognosis of CAG recently. In this study, we have found that the prognosis of clinical symptoms was better in the CAG without the infection of H. pylori, which proved that the clinical treatment of CAG should pay special attention to the infection of H. pylori.
As expected, macrophage is an important innate immune cell and makes up the majority of leukocytes [23]. It can play an important role in fighting pathogenic microorganisms and repairing the damage, in which there are two primary types of macrophages based on the expression of CD11C with the changes of micro-environments [24]. In this condition, M1 macrophages can help the host defend against the invasion of foreign microorganisms, but continuous activation could cause the chronic inflammation. Meanwhile, the polarization of M2 macrophages can play an anti-inflammatory role. However, in vivo and in vitro experiments confirmed that M1 macrophages were not easy polarizing to M2 macrophages [25, 26]. In this case, the cytokines that are released by inflammatory cells include the TNF-α, INF-γ, IL-6, monocyte chemoattractant protein 1 (MCP-1), and IL-1. All these molecules may act on the immune cells leading to local and generalized inflammation. Adversely, the cytokines such as Arg-1, IL-4 and IL-10 can be secreted by M2 macrophages [23-26].
With the deepening researches, the sustained inflammatory reaction and abnormal apoptosis of gastric mucosa are confirmed as important causes in the development and pathogenesis of CAG, which had been drawn by mounting evidence from many researchers [27, 28]. However, the exact mechanism still remains unclear. It is well established that the development of CAG is characterized by immune cell infiltration and low-grade inflammation. Based on the above results, we demonstrated that the infections of H. pylori could aggravate the inflammatory response and polarization of M1/M2 macrophage, with much higher pro-inflammatory (iNOS, TNF-α, IFN-γ, and IL-6) and lower anti-inflammatory cytokines (Arg-1, IL4 and IL-10). Meanwhile, negative correlations were shown between the expressions of iNOS, TNF-α, IFN-γ and IL-6, and the prognosis of CAG patients, while positive correlations were observed between the expressions of IL-4 and IL-10, and the prognosis of CAG patients. Nevertheless, to our knowledge, the main limitation of our study was its small sample size, which could decrease our power to find the significant associations between the H. pylori infection and macrophage polarization. Therefore, our results still need to be further confirmed by much larger studies in the future. Another limitation was that few patients agreed to undergo the examination of gastroscopy after the eradication of H. pylori, as the gastroscopy was still an invasive examination, so it is not possible to obtain the gastric mucosa for the analysis of macrophage polarization.
In summary, H. pylori infection could affect the prognosis of CAG, and there were significant correlations between the prognosis of CAG and the disorders of pro-inflammatory and anti-inflammatory cytokines such as the M1/M2 macrophage polarization, which could provide the corresponding support for our clinical intervention that more attention should be paid to the situation of H. pylori infection in the process of CAG.
Acknowledgments
None to declare.
Financial Disclosure
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
The written informed consent from each subject was also obtained before they were agreed to involve in this survey.
Author Contributions
CZ and LX contributed equally as the co-first authors. They had full access to all of the study data, and take responsibility for the integrity of data analysis. LX did the study concept and design. CZ drafted the manuscript. YZ gave the administrative, technical, and did the study supervision.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author for reasonable request.
| References | ▴Top |
- Massironi S, Zilli A, Elvevi A, Invernizzi P. The changing face of chronic autoimmune atrophic gastritis: an updated comprehensive perspective. Autoimmun Rev. 2019;18(3):215-222.
doi pubmed - Neumann WL, Coss E, Rugge M, Genta RM. Autoimmune atrophic gastritis—pathogenesis, pathology and management. Nat Rev Gastroenterol Hepatol. 2013;10(9):529-541.
doi pubmed - Du Y, Bai Y, Xie P, Fang J, Wang X, Hou X, Tian D, et al. Chronic gastritis in China: a national multi-center survey. BMC Gastroenterol. 2014;14:21.
doi pubmed pmc - Li Y, Xia R, Zhang B, Li C. Chronic atrophic gastritis: a review. J Environ Pathol Toxicol Oncol. 2018;37(3):241-259.
doi pubmed - Holleczek B, Schottker B, Brenner H. Helicobacter pylori infection, chronic atrophic gastritis and risk of stomach and esophagus cancer: Results from the prospective population-based ESTHER cohort study. Int J Cancer. 2020;146(10):2773-2783.
doi pubmed - Lahner E, Carabotti M, Annibale B. Treatment of Helicobacter pylori infection in atrophic gastritis. World J Gastroenterol. 2018;24(22):2373-2380.
doi pubmed pmc - Ito N, Tsujimoto H, Ueno H, Xie Q, Shinomiya N. Helicobacter pylori-mediated immunity and signaling transduction in gastric cancer. J Clin Med. 2020;9(11):3699.
doi pubmed pmc - Blosse A, Lehours P, Wilson KT, Gobert AP. Helicobacter: inflammation, immunology, and vaccines. Helicobacter. 2018;23(Suppl 1):e12517.
doi pubmed pmc - Yang T, Wang R, Liu H, Wang L, Li J, Wu S, Chen X, et al. Berberine regulates macrophage polarization through IL-4-STAT6 signaling pathway in Helicobacter pylori-induced chronic atrophic gastritis. Life Sci. 2021;266:118903.
doi pubmed - Retraction: TLR4-dependent NF-kappaB activation and mitogen- and stress-activated protein kinase 1-triggered phosphorylation events are central to helicobacter pylori peptidyl prolyl cis-, trans-Isomerase (HP0175)-mediated induction of IL-6 release from macrophages. J Immunol. 2015;195(4):1902.
doi pubmed - Che Y, Geng B, Xu Y, Miao X, Chen L, Mu X, Pan J, et al. Helicobacter pylori-induced exosomal MET educates tumour-associated macrophages to promote gastric cancer progression. J Cell Mol Med. 2018;22(11):5708-5719.
doi pubmed pmc - Kong H, You N, Chen H, Teng YS, Liu YG, Lv YP, Mao FY, et al. Helicobacter pylori-induced adrenomedullin modulates IFN-gamma-producing T-cell responses and contributes to gastritis. Cell Death Dis. 2020;11(3):189.
doi pubmed pmc - Faass L, Stein SC, Hauke M, Gapp M, Albanese M, Josenhans C. Contribution of heptose metabolites and the cag pathogenicity island to the activation of monocytes/macrophages by helicobacter pylori. Front Immunol. 2021;12:632154.
doi pubmed pmc - Masi A, DeMayo MM, Glozier N, et al. Trial on treatment and typing or grading chronic gastritis under endoscopies. Chin J Dig Dis. 2004;21:77-78.
- Keren D, Matter I, Rainis T, Goldstein O, Stermer E, Lavy A. Sleeve gastrectomy leads to Helicobacter pylori eradication. Obes Surg. 2009;19(6):751-756.
doi pubmed - Szlachcic A. The link between Helicobacter pylori infection and rosacea. J Eur Acad Dermatol Venereol. 2002;16(4):328-333.
doi pubmed - Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424.
doi pubmed - Ohata H, Kitauchi S, Yoshimura N, Mugitani K, Iwane M, Nakamura H, Yoshikawa A, et al. Progression of chronic atrophic gastritis associated with Helicobacter pylori infection increases risk of gastric cancer. Int J Cancer. 2004;109(1):138-143.
doi pubmed - Salvatori S, Marafini I, Laudisi F, Monteleone G, Stolfi C. Helicobacter pylori and gastric cancer: pathogenetic mechanisms. Int J Mol Sci. 2023;24(3):2895.
doi pubmed pmc - Sukri A, Hanafiah A, Mohamad Zin N, Kosai NR. Epidemiology and role of Helicobacter pylori virulence factors in gastric cancer carcinogenesis. APMIS. 2020;128(2):150-161.
doi pubmed - Shah SC, Piazuelo MB, Kuipers EJ, Li D. AGA clinical practice update on the diagnosis and management of atrophic gastritis: expert review. Gastroenterology. 2021;161(4):1325-1332.e1327.
doi pubmed pmc - Cai Q, Shi P, Yuan Y, Peng J, Ou X, Zhou W, Li J, et al. Inflammation-associated senescence promotes helicobacter pylori-induced atrophic gastritis. Cell Mol Gastroenterol Hepatol. 2021;11(3):857-880.
doi pubmed pmc - Anderson NR, Minutolo NG, Gill S, Klichinsky M. Macrophage-Based Approaches for Cancer Immunotherapy. Cancer Res. 2021;81(5):1201-1208.
doi pubmed - Xue J, Cheng Y, Hao HJ. Advances in the immunomodulatory effects of mesenchymal stem cells on macrophages. Journal of PLA Medical College. 2018;39:353-355.
- Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, Seifi B, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233(9):6425-6440.
doi pubmed - Wu K, Lin K, Li X, Yuan X, Xu P, Ni P, Xu D. Redefining tumor-associated macrophage subpopulations and functions in the tumor microenvironment. Front Immunol. 2020;11:1731.
doi pubmed pmc - Jeong M, Park JM, Han YM, Kangwan N, Kwon SO, Kim BN, Kim WH, et al. Dietary intervention of artemisia and green tea extracts to rejuvenate helicobacter pylori-associated chronic atrophic gastritis and to prevent tumorigenesis. Helicobacter. 2016;21(1):40-59.
doi pubmed - Li W, Chen M, Xu L, Lv Z, Chen L, Li Y, He W. Morroniside alleviates coxsackievirus B3-induced myocardial damage apoptosis via restraining NLRP3 inflammasome activation. RSC Adv. 2019;9(3):1222-1229.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


