| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Original Article
Volume 16, Number 4, August 2023, pages 203-208
Sex and Race Disparities in Hepatocellular Carcinoma Surveillance in Patients With Chronic Hepatitis B During COVID-19: A Single-Center Retrospective Review
William S. Reichea, h, Stephen Coopera, Christopher J. Destacheb, c, Suhail Sidhud, Bryce Schuttea, Darby Keirnsd, Elezabeth Mace, Ian Ngf, Haitam Buaishag, Manasa Velagapudic
aDepartment of Medicine, CHI Creighton University Medical Center, Omaha, NE, USA
bDepartment of Pharmacy Practice, Creighton University School of Pharmacy and Health Professions, Omaha, NE, USA
cDivision of Infectious Diseases, CHI Creighton University Medical Center, Omaha, NE, USA
dCreighton University School of Medicine, Omaha, NE, USA
eCommonSpirit Health Specialty Pharmacy, Phoenix, AZ, USA
fDepartment of Clinical Research and Public Health, Creighton University School of Medicine, CHI Creighton University Medical Center, Omaha, NE, USA
gDivision of Gastroenterology and Hepatology, CHI Creighton University Medical Center, Omaha, NE, USA
hCorresponding Author: William S. Reiche, Department of Medicine, CHI Creighton University Medical Center, Omaha, NE, USA
Manuscript submitted March 12, 2023, accepted May 2, 2023, published online July 12, 2023
Short title: Sex and Race Disparities in HCC Surveillance
doi: https://doi.org/10.14740/gr1614
| Abstract | ▴Top |
Background: The management of patients with chronic hepatitis B (CHB) is complex and spans multiple medical specialties. As a result of this complexity, patients with CHB often do not receive adequate monitoring including hepatocellular carcinoma (HCC) surveillance with abdominal ultrasonography. Previous studies have identified multiple factors associated with decreased HCC surveillance. We aimed to identify the impact of race and sex on HCC surveillance in patients with CHB.
Methods: We performed a single health system chart review between January 2018 and January 2022. Differences between sex and race were evaluated using the Chi-square test and Fisher’s exact test, and continuous variables were analyzed using analysis of variance (ANOVA).
Results: A total of 248 patient records between January 2018 and January 2022 were evaluated. In total 37% of females were adequately screened for HCC in any of the 6-month time frames compared to 26% of males. During the coronavirus disease 2019 (COVID-19) surge, surveillance rates were reduced in both men and women. During the first 6 months of the COVID-19 surge, there was a significant difference in screening between men and women (19% vs. 35%, P = 0.026). There was a decrease in HCC screening across all races during the COVID-19 surge; however, no significant difference when comparing races was found.
Conclusion: Men received less HCC surveillance compared to women. These differences were more pronounced during the COVID-19 pandemic surge. Obtaining appropriate surveillance is important and retrospective evaluations can help us determine the presence of health-related social needs so that progress can be made toward achieving health equity.
Keywords: Sex; Race; Chronic hepatitis B; HCC; Surveillance
| Introduction | ▴Top |
Chronic hepatitis B (CHB), defined as a positive hepatitis B surface antigen, affects approximately 296 million worldwide [1]. Patients are often not identified as having CHB until they become symptomatic or unless they receive a screening test. Despite a high prevalence, only 70% of patients are aware of hepatitis B virus (HBV) infection status in the United States [2]. Additionally, less than half of patients with suspected or diagnosed CHB are receiving care consistent with most recent guidelines of American Association for the Study of Liver Diseases (AASLD) [3]. While indolent, the long-term sequelae of CHB include cirrhosis and hepatocellular carcinoma (HCC) which can have devastating consequences. Abdominal ultrasound (AUS) surveillance is fundamental in early detection and management of these complications [3]. Gaps in diagnosing, continuity of care, and treating CHB infection have been attributed to unfamiliarity with guidelines, the indolent nature of infection, and socioeconomic factors [4]. While specialty care by gastroenterology (GI) and/or infectious diseases (IDs) has been associated with improved outcomes, there are still significant lapses in the treatment of CHB and in HCC surveillance [5-7].
CHB occurs most commonly because of HBV infection during childhood and less likely because of exposure and acute infection in adulthood. It is well established that men who have sex with men, patients with intravenous drug use, healthcare workers, incarcerated individuals, international travelers, patients presenting from endemic regions, and those who have human immunodeficiency virus (HIV) infection, hepatitis C, and/or chronic liver disease are at increased risk of hepatitis B infection [8, 9]. However, more information is needed to determine the precise role of socioeconomic factors in CHB treatment, hepatitis B vaccination, and rates of HCC surveillance in patients with CHB. In this study, we aimed to determine the impact of race and sex on rates of HCC surveillance.
| Materials and Methods | ▴Top |
Protocol
This single-center retrospective cohort study was approved as exempt by the Institutional Review Board at Creighton University (InfoEd record number: 2002669), and has been conducted in accordance with the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) statement for key items which should be included in cohort studies (Supplementary Material 1, www.gastrores.org). The study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
We conducted a retrospective chart review of patients treated for CHB in outpatient GI and ID clinics at CHI Health CUMC-Bergan Mercy Hospital in Omaha, Nebraska between January 2018 and January 2022. Patients were included if they were at least 19 years of age and had a positive hepatitis B surface antigen (HbSAg) test. Patients were excluded if they were not adults (< 19 years old) or died within 6 months of initial HbSAg test. Patients were stratified via department follow-up ID clinic, GI clinic, not in clinic, and both ID/GI clinic. HCC surveillance was further stratified by 6-month time intervals throughout the study period to compare surveillance during the coronavirus disease 2019 (COVID-19) surge at our region and after the surge. The COVID-19 pandemic surge was defined as approximately January 1, 2020 to June 30, 2021 for Nebraska. Patients who received a diagnosis of CHB within the first 6 months of the study period were not studied during the first 6-month interval as HCC surveillance was not indicated. Patients were considered lost to follow-up if they did not see an ID or GI doctor for surveillance in clinic following their initial HbsAg positive test result.
Baseline patient characteristics including age, sex, race, ethnicity, non-English speaking, insurance status and whether patients were seen in GI or ID clinics were collected. Laboratory data collected included hepatitis B virus DNA, hepatitis B e antigen, hepatitis C antibody, hepatitis A total antibody, and alanine aminotransferase (ALT). Additional data on family history of HCC, hepatitis A vaccination status, and assessment of liver fibrosis were obtained. Fibrosis-4 score, aspartate aminotransferase to platelet ratio index (APRI), FibroSure, Fibroscan, liver biopsy or presence of cirrhosis on imaging were used to assess liver fibrosis. The primary outcome studied was determining if HCC surveillance was indicated and/or performed according to the AASLD practice guidelines. HCC surveillance was deemed completed if abdominal imaging (AUS/computed tomography (CT) scan) was performed within a 6-month interval of time.
Statistical analyses
Data were collected using Excel spreadsheet (Microsoft, Inc., Pullman, WA) and then imported into SPSS (Ver 28, IBM, Inc.). Categorical variables, whether patients received appropriate HCC screening, were analyzed using Chi-square or Fisher’s exact test. Continuous variables were analyzed using analysis of variance (ANOVA). A significant P-value was determined as P < 0.05.
| Results | ▴Top |
From January 2018 through January 2022, a total of 248 patients with diagnoses of CHB were treated at CHI Bergan Mercy ID and GI clinics. A total of 76 of 248 patients met inclusion criteria with CHB and need for surveillance (Fig. 1). Of the 76 patients, 56 (73.6%) were male and 20 (26.3%) were female. Majority of patients were Asian (48, 63.2%) followed by Black (17, 22.4%) and White (6, 7.9%) races (Table 1). There were 22 patients who did not see either an ID or GI doctor in clinic during the study period: 14 (63.6%) of these patients were men and eight (36.4%) were women. Stratifying by race, 12 (54.5%) were Asian, nine (40.9%) were Black and one (4.5%) was White.
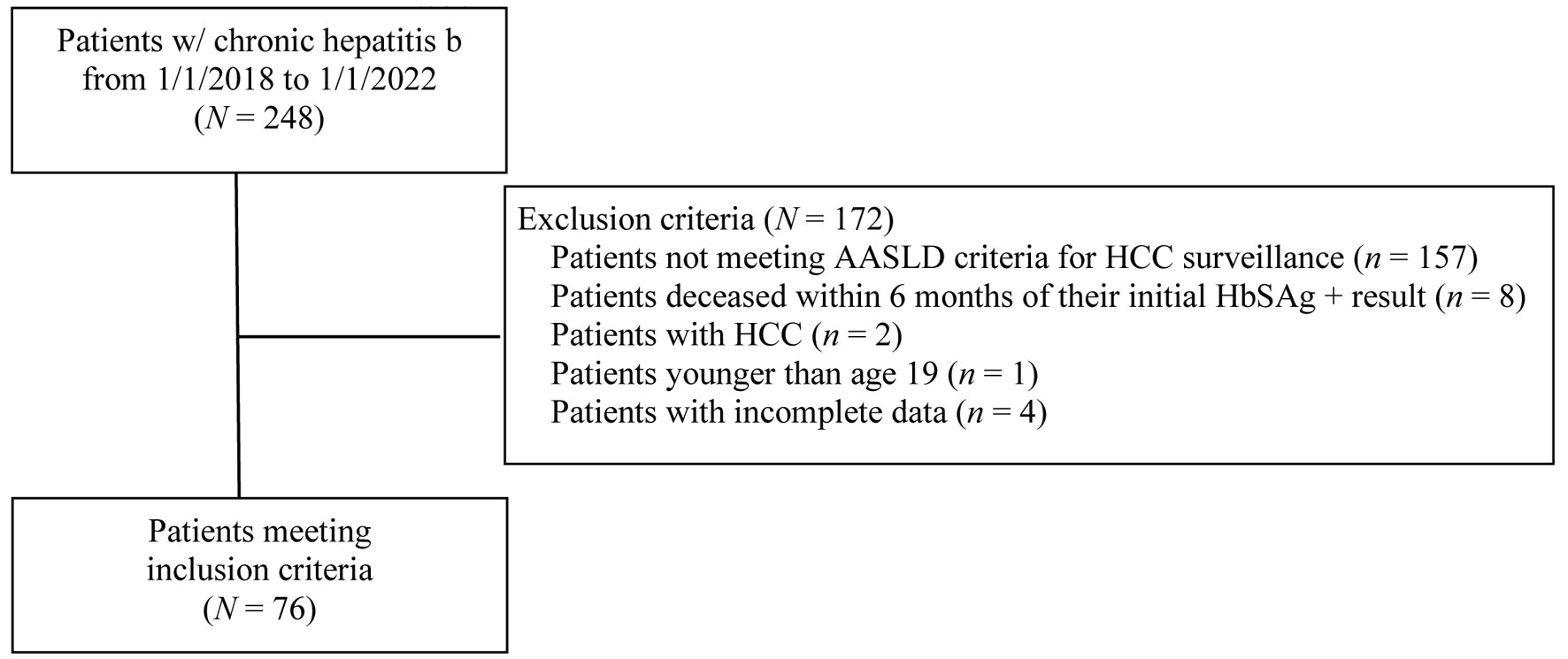 Click for large image | Figure 1. Patient flowchart for inclusion and exclusion criteria. AASLD: American Association for the Study of Liver Diseases; HCC: hepatocellular carcinoma; HbSAg: hepatitis B surface antigen. |
 Click to view | Table 1. Patient Demographics |
Throughout the entire study period, AUS was completed in 37% of the opportunities for surveillance in women and 26% in men (Table 2). During non-surge time, HCC screening occurred 62% of the time in men and 53% of the time in women. During the pandemic, HCC surveillance decreased in both men and women with a more pronounced relative decrease in screening men (38%) compared to women (47%) (Table 3). It is notable during the first 6-month interval of the pandemic there was a statistically significant difference in screening between men and women (19% for men and 35% for women, P = 0.026).
 Click to view | Table 2. HCC Surveillance Data Stratified by 6-Month Intervals Throughout the Study Period and Sex |
 Click to view | Table 3. HCC Surveillance Data Before or During the COVID-19 Pandemic |
Rates of HCC screening in non-surge time were similar across all races: 25.5-40.9% for Asian, 22-60% for Black, and 40-66.7% for White (Table 4). While not statistically significant when compared to non-pandemic time, HCC screening rates decreased across all races during the COVID-19 surge: 10-28.6% for Asian, 18.2-45.5% for Black, and 16.7-33.3% for White. During the COVID-19 surge, 20% of Asians, 34% of Black and 22% of White patients received an AUS (P = 0.069).
 Click to view | Table 4. HCC Surveillance Data Stratified by 6-Month Intervals Throughout the Study Period and Race |
| Discussion | ▴Top |
To the best of our knowledge, we believe our study is the first to identify disparities in HCC screening in patients with CHB with men receiving less adequate screening compared to women. We found that men received HCC surveillance only 26% of the time compared to 36% of the time in women. Although statistically non-significant, men were more likely to have complete loss of follow-up after a diagnosis of CHB. No statistically significant disparities in race were identified when comparing patients of Asian, Black, or White race. The COVID-19 pandemic did result in non-significant reductions in HCC screening across all sex and race groups. During the first 6-month interval of the pandemic, there was a significant difference in screening with men only being appropriately screened 19% of the time compared to 35% in women.
Prior studies have looked to elucidate possible reasons for disparities in HCC screening rates including advanced age, insurance status, provider specialty (GI vs. ID vs. non-specialty), and socioeconomic factors [5, 6, 10]. Similarly, sex and race have been investigated as possible factors contributing to disparities in HCC surveillance. Most recently Parikh et al studied the impact of race and sex on HCC screening in patients with CHB in a multicenter study in 2022 [11]. Their study population was largely comprised of white men and only 14 of 629 patients were of Asian race. They concluded semiannual surveillance for HCC was inversely associated with Black race. Additionally, Singal et al studied the impact of race and sex on HCC surveillance in patients with cirrhosis [12]. Their population was also largely comprised of men, roughly 65% of their 904 patients studied. Singal et al found that race was associated with inconsistent surveillance and that consequently men were more likely to receive surveillance.
The differences observed in our study may be due to several factors. Our study was a single-center retrospective review like Singal et al, while Parikh et al had a multicentric cohort. There may be site level variation in surveillance attainment as biogeographical factors and other social determinants of health, including gender and sex, have been found to effect perceptions and completion of cancer screening in the past [13, 14]. While there were no statistically significant differences in race in our study, we have incorporated more patients with Asian and Black race. Forty-eight patients (63.2% of all patients) with Asian race were represented in our study, while less than 20 patients of Asian race were included in each of the previously mentioned larger studies. The additive effect of factors such as race, sex, and numerous others could be contributing to the differences we observed. Importantly, surveillance rates were noted to be overall much lower in the 2015 study which may reflect lag in implementing evidence-based practice [15]. It is unclear if the overall decreased rates contributed to the race and sex disparities noted. Study interval differences may have been an additional factor which contributed to our findings; our study interval was inclusive of the COVID-19 surge in Omaha, Nebraska. The reason for the statistically significant sex difference which occurred during the first 6-month interval of the pandemic is unclear.
We recognize while identifying single areas of disparities is important, it may not be truly representative of the real-world multifactorial disparities which exist. Regardless, we believe the findings of our study have significant clinical implications. It is important to identify that men may be at higher risk of inadequate HCC surveillance than women. It is important to note men of Asian and Black race were most heavily represented in the patients that had a diagnosis of CHB but were not evaluated at any time in the clinic for this condition. While the incidence of HCC has stabilized in recent years, primary liver cancers (75% of which are HCC) are associated with the third highest cancer mortality worldwide [16, 17]. HCC surveillance in the setting of CHB is even more important as HBV-related deaths are expected to peak by 2035 [1]. Men are three to four times more likely than women to develop liver cancer, and current guidelines reflect this increased risk especially in Asian and Black men as surveillance is recommended to start in 6-month intervals at age 40 [5, 14]. Guidelines for HCC surveillance also call for surveillance in Asian women after age 50 and in patients with cirrhosis, patients who have a first-degree relative with HCC, or if patients have concomitant hepatitis D virus infection. The importance of HCC surveillance is well described. HCC may be diagnosed much earlier with surveillance, often HCC manifests only with symptoms in advanced disease. HCC surveillance has been associated with improved survival and treatment. Additionally, if HCC does develop, it may be diagnosed and intervened upon at an earlier stage [12].
Our study does have several limitations. Our sample size was small limiting statistical power and the ability to perform logistic regression. This is a single-center study which may limit the generalizability of results to other centers. However, larger multicentric studies may miss site-specific differences that need to be considered. The COVID-19 surge is loosely defined to include the period during which COVID-19-related deaths, healthcare strain, and fear of COVID-19 had the greatest impact on healthcare. This time may vary when compared broadly based on geographic location; however, the trends represented likely amplify the baseline disparities that existed during non-COVID surge. Finally, our results did not make the distinction between HCC ordering and completion, and we only considered completion.
Our findings pose a new research area that will require further work. The singular impact of one socioeconomic effect or identifier like race and sex may not provide ample information for decision-making regarding likelihood of HCC surveillance completion. Efforts are needed to create predictive models which will incorporate multiple socioeconomic factors (race, sex, biogeographical impact) as the synergistic effect of these factors is more likely to represent real-world occurrence. Moreover, methods to improve surveillance rates in high-risk groups need to be developed. Patient engagement has been noted to improve the likelihood of surveillance [18]. Using mobile support tools increases communication between patients and providers and has been found to increase rates of HBV screening, and similar improvement may be achieved for HCC screening if implemented [19]. Mobile support tools may additionally be used to facilitate scheduling and grouping appointments to reduce the burden of transportation and time.
Along with cost-effectiveness and radiologic capacity, newer technologies being developed for HCC surveillance must consider health-related social needs in determining feasibility [20].
In conclusion, our retrospective cohort study which was comprised of most patients with Asian race and men identified sex disparities in AUS completion for HCC surveillance. When indicated, men had lower rates of surveillance compared to women. Our study highlights the need to focus interventions to improve HCC surveillance in men of Black and Asian race as these patients had the lowest surveillance rates and were most frequently lost to follow-up. We recommend future, multi-center studies be conducted to better understand the disparities in CHB and to better achieve health equity.
| Supplementary Material | ▴Top |
Suppl 1. STROBE Statement - Checklist of Items That Should Be Included in Reports of Observational Studies.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Not applicable.
Author Contributions
MV and HB designed the study. WR, SC, BS, SS, and DK collected data. CD analyzed the data. WR, SC, CD, and SS wrote the first draft of the paper. All authors contributed to writing, reviewing, and editing, and approved the final version of the manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
AASLD: American Association for the Study of Liver Diseases; AUS: abdominal ultrasound; CHB: chronic hepatitis B; HCC: hepatocellular carcinoma; GI: gastroenterology; ID: infectious disease; STROBE: Strengthening the Reporting of Observational Studies in Epidemiology
| References | ▴Top |
- Dusheiko G, Agarwal K, Maini MK. New approaches to chronic hepatitis B. N Engl J Med. 2023;388(1):55-69.
doi pubmed - Wong RJ, Brosgart CL, Welch S, Block T, Chen M, Cohen C, Kim WR, et al. An updated assessment of chronic hepatitis B prevalence among foreign-born persons living in the United States. Hepatology. 2021;74(2):607-626.
doi pubmed pmc - Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS, Jr., et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67(4):1560-1599.
doi pubmed pmc - Ye Q, Kam LY, Yeo YH, Dang N, Huang DQ, Cheung R, Nguyen MH. Substantial gaps in evaluation and treatment of patients with hepatitis B in the US. J Hepatol. 2022;76(1):63-74.
doi pubmed - Guo A, Pomenti S, Wattacheril J. Health disparities in screening, diagnosis, and treatment of hepatocellular carcinoma. Clin Liver Dis (Hoboken). 2021;17(5):353-358.
doi pubmed pmc - Kim AK, Singal AG. Health disparities in diagnosis and treatment of hepatocellular carcinoma. Clin Liver Dis (Hoboken). 2014;4(6):143-145.
doi pubmed pmc - Tran S, Jeong D, Henry L, Cheung RC, Nguyen MH. Initial evaluation, long-term monitoring, and hepatocellular carcinoma surveillance of chronic hepatitis B in routine practice: a Nationwide US study. Am J Gastroenterol. 2021;116(9):1885-1895.
doi pubmed - Zhang X, Guan L, Tian H, Zeng Z, Chen J, Huang D, Sun J, et al. Risk factors and prevention of viral hepatitis-related hepatocellular carcinoma. Front Oncol. 2021;11:686962.
doi pubmed pmc - Smith JM, Uvin AZ, Macmadu A, Rich JD. Epidemiology and treatment of hepatitis B in prisoners. Curr Hepatol Rep. 2017;16(3):178-183.
doi pubmed pmc - McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology. 2021;73(Suppl 1):4-13.
doi pubmed pmc - Parikh ND, Tayob N, Al-Jarrah T, Kramer J, Melcher J, Smith D, Marquardt P, et al. Barriers to surveillance for hepatocellular carcinoma in a multicenter cohort. JAMA Netw Open. 2022;5(7):e2223504.
doi pubmed pmc - Singal AG, Li X, Tiro J, Kandunoori P, Adams-Huet B, Nehra MS, Yopp A. Racial, social, and clinical determinants of hepatocellular carcinoma surveillance. Am J Med. 2015;128(1):90.e91-97.
doi pubmed pmc - Davis JL, Buchanan KL, Katz RV, Green BL. Gender differences in cancer screening beliefs, behaviors, and willingness to participate: implications for health promotion. Am J Mens Health. 2012;6(3):211-217.
doi pubmed pmc - Guo Y, Szurek SM, Bian J, Braithwaite D, Licht JD, Shenkman EA. The role of sex and rurality in cancer fatalistic beliefs and cancer screening utilization in Florida. Cancer Med. 2021;10(17):6048-6057.
doi pubmed pmc - Rubin R. It takes an average of 17 years for evidence to change practice-the burgeoning field of implementation science seeks to speed things up. JAMA. 2023;329(16):1333-1336.
doi pubmed - American Cancer Society. Cancer Facts & Figures. 2023. Accessed November 15, 2022. https://www.cancer.org/research/cancer-facts-statistics.html.
- Van Ryn M. Avoiding unintended bias: strategies for providing more equitable health care. Minn Med. 2016;99(2):40-46.
pubmed pmc - Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med. 2014;11(4):e1001624.
doi pubmed pmc - Khalili M, Kim NJ, Tsoh JY, Walsh JME, Goldman LE, Gildengorin G, Wong C, et al. Health within reach-a patient-centered intervention to increase hepatitis B screening among Asian Americans: a randomized clinical trial. J Gen Intern Med. 2022;37(13):3242-3250.
doi pubmed pmc - Singal AG, Reig M, Villanueva A. Emerging tools for hepatocellular carcinoma surveillance. Am J Gastroenterol. 2022;117(12):1948-1951.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


