| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Original Article
Volume 16, Number 2, April 2023, pages 105-117
Incidence and Cross-Continents Differences in Endoscopic Retrograde Cholangiopancreatography Outcomes Among Patients With Cirrhosis: A Systematic Review and Meta-Analysis
Saqr Alsakarneha, h, Fouad Jabera, h, i, Khalid Ahmedb, Fares Ghanemc, Wael T. Mohammada, Mohamed K. Ahmeda, Mohamad Khaled Almujarkeshd, Thomas Biermane, f, John Campbelle, f, Yazan Abboudg, Muhammad Shah Mirana, John H. Helzberge, f, Hassan M. Ghoze
aDepartment of Internal Medicine, University of Missouri-Kansas City, Kansas City, MO, USA
bDepartment of Internal Medicine, The Wright Center for Graduate Medical Education, Scranton, PA, USA
cDepartment of Internal Medicine, East Tennessee State University, Johnson City, TN, USA
dDepartment of Internal Medicine, Wayne State University/Detroit Medical Center, Detroit, MI, USA
eDivision of Gastroenterology, University of Missouri-Kansas City, Kansas City, MO, USA
fDivision of Gastroenterology and Hepatology, Saint Luke’s Hospital, Kansas City, MO, USA
gDepartment of Internal Medicine, Rutgers New Jersey Medical School, NJ, USA
hThese authors contributed equally to the study.
iCorresponding Author: Fouad Jaber, Department of Internal Medicine, University of Missouri-Kansas City, Kansas City, MO, USA
Manuscript submitted March 5, 2023, accepted March 28, 2023, published online April 28, 2023
Short title: ERCP Outcomes in Patients With Cirrhosis
doi: https://doi.org/10.14740/gr1610
| Abstract | ▴Top |
Background: There are conflicting data on the frequency and variability of endoscopic retrograde cholangiopancreatography (ERCP) outcomes in patients with cirrhosis. Our aim was to systematically review the literature on the incidence of post-ERCP adverse events in cirrhotic patients and to examine the differences across continents.
Methods: We searched PubMed/MEDLINE, EMBASE, Scopus, and Cochrane databases to identify studies reporting adverse events after ERCP in patients with cirrhosis from conception to September 30, 2022. The random effects model was used to calculate odds ratios (ORs), mean differences (MDs), and confidence intervals (CIs). A P value < 0.05 was considered statistically significant. Heterogeneity was assessed using the Cochrane Q-statistic (I2).
Results: Twenty-one studies that included 2,576 cirrhotic patients and 3,729 individual ERCPs were analyzed. The pooled overall rate of adverse events after ERCP in patients with cirrhosis was 16.98% (95% CI: 13.06-21.29%, P < 0.001, I2 = 86.55%). ERCPs performed in Asia had the highest ERCP adverse events with an overall complication rate of 19.90%, while the lowest overall adverse events were in North America at 13.04%. The pooled post-ERCP bleeding, pancreatitis, cholangitis and perforation were 5.10% (95% CI: 3.33-7.19%, P < 0.001, I2 = 76.79%), 3.21% (95% CI: 2.20-5.36%, P = 0.03, I2 = 42.25%), 3.02% (95% CI: 1.19-5.52%, P < 0.001, I2 = 87.11%), and 0.12% (95% CI: 0.00 - 0.45, P = 0.26, I2 = 15.76%), respectively. The pooled post-ERCP mortality rate was 0.22% (95% CI: 0.00-0.85%, P = 0.01, I2 = 51.86%).
Conclusions: This meta-analysis shows that the overall complication rates after ERCP, bleeding, pancreatitis, and cholangitis are high in patients with cirrhosis. Because cirrhotic patients are more likely to have post-ERCP complications, with significant cross-continent variations, the risks and benefits of ERCP in this patient population should be carefully considered.
Keywords: ERCP; Liver cirrhosis; Meta-analysis; ERCP complications; Adverse events
| Introduction | ▴Top |
Endoscopic retrograde cholangiopancreatography (ERCP) is a key endoscopic procedure for diagnosing and treating biliary and pancreatic diseases [1]. There has been robust growth in the use of ERCP in the United States and worldwide [2]. Common indications for the use of ERCP are obstructive jaundice, treatment of biliary or pancreatic duct system disease, tissue sampling, pancreatic cancer, choledocholithiasis, and cholangiocarcinoma [1].
ERCP utilization trends vary significantly in the cirrhotic population compared to the general population [2, 3]. Gallstones and choledocholithiasis are more common in patients with cirrhosis and may require frequent ERCP interventions [4, 5]. Inherently, ERCP carries risks of complications, including post-ERCP pancreatitis (PEP), bleeding, infection, and perforation [6]. It is believed that the risk of these adverse events is higher in cirrhotic patients undergoing ERCP [7]. This could be attributed to poor synthetic function in this cohort and the resulting portal hypertension, ascites, varices, coagulopathy, and encephalopathy [8].
Although the risks of ERCP in patients with cirrhosis are recognized, there is conflicting literature on the outcomes of ERCP in this cohort. One meta-analysis has examined this issue [7], but recent studies have reported inconsistent results [9-16]. A definitive conclusion is yet to be established. Therefore, we performed this systematic review and meta-analysis to assess ERCP adverse events, mortality outcomes, and intercontinental variation. The primary endpoints are ERCP-related adverse events, including: 1) bleeding; 2) PEP; 3) cholangitis; and 4) perforation. Secondary outcomes included analysis of mortality rates and cross-continental differences in these adverse events.
| Materials and Methods | ▴Top |
The preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement guidelines were followed [17].
Search strategy and selection criteria
A comprehensive online literature search was performed in the PubMed/MEDLINE, Scopus, EMBASE, and Cochrane databases using the keywords: “ERCP”, “endoscopic retrograde cholangiopancreatography”, “complications”, “adverse events”, and “cirrhosis of the liver” in various combinations from inception to September 30, 2022, for studies reporting post-ERCP complications in cirrhotic patients. We also attempted to find articles that may have been missing in the literature search by manually searching the reference lists of all candidate articles as well as previous meta-analyses. The search was restricted to human studies, with no restrictions on region, publication type, or language. Patient consent and Institutional Review Board (IRB) approval were not required as the meta-analysis studies did not include human subjects.
Inclusion and exclusion criteria
Inclusion criteria were: 1) a well-defined prospective, cohort, retrospective, case-control, or clinical study (including randomized controlled trials); 2) studies that reported data sufficient to calculate event rate, relative risk (RR) or odds ratio (OR); 3) studies evaluating ERCP complications in patients with cirrhosis; 4) studies comparing post-ERCP complications in cirrhotic and non-cirrhotic patients. Several studies were excluded for one of the following reasons: 1) they included only patients without cirrhosis; 2) providing insufficient information about the number of ERCPs or their outcome to calculate the event rate, RR, or OR for our main results; or 3) case studies, editorials, opinions, letters to the editor, animal studies, or meta-analysis.
Study selection and data extraction
First, we searched the databases for studies performed on human subjects that described post-ERCP complications in patients with cirrhosis. Two investigators (SA and FJ) independently reviewed each title and abstract based on our inclusion and exclusion criteria. All studies that passed the first screening process were thoroughly reviewed and assessed in the second screening phase. We selected the studies that only reported post-ERCP complications in patients with cirrhosis and studies that compared the RR or OR in patients with cirrhosis. Data from eligible studies were extracted into a standardized table for analysis.
Quality assessment
Using the Newcastle-Ottawa Scale (NOS) [18], the methodological quality of the included cohort studies was assessed independently by two investigators. A third author addressed each discrepancy by reviewing the original article. Risk of bias was assessed by including cirrhotic patient population identification, outcome identification, and potential confounders in the analysis. Points (maximum 9 points) were assigned to each cohort using a checklist that had been developed. Studies with more than 6 points were considered to be of good quality; those with 5 - 6 points were considered studies of reasonable quality, and those with < 5 points were studies of poor quality.
Data synthesis and statistical analysis
STATA Version 17.0 (StataCorp LLC, College Station, Texas, USA) was used for the statistical analysis. Using the Freeman-Turkey double arcsine transformation (FTT) method, the pooled weight-adjusted event rate estimate for the clinical outcomes in each group was calculated using STATA’s Metaprop package. Between-study heterogeneity was assessed using the Cochrane Q-statistic (I2), which represents the percentage of total between-study variation that cannot be attributed solely to chance. Between-study heterogeneity was scored as low if 25% < I2 ≤ 50%, moderate if 50% < I2 ≤ 75%, and high if I2 > 75%. When significant heterogeneity was found, we used subgroup analysis, sensitivity analysis, and meta-regression to explore the origin of the heterogeneity. Subgroup analyses were performed to determine whether the complication rate was influenced by 1) study design (i.e., single-center versus multi-center studies); and 2) study region (i.e., Europe, North America, South America, or Asia). The Egger’s regression test and funnel plots reporting study-specific ORs and standard errors of the logarithm (OR) were performed to further investigate the presence of publication bias. The Egger’s test with a P value < 0.05 was considered to have statistically significant publication bias. In addition, the asymmetry of the funnel plots indicates a possible publication bias. In addition to the ethical standards of the institution responsible for people, this meta-analysis was conducted in compliance with the Helsinki Declaration.
| Results | ▴Top |
Search results
We initially identified a total of 872 unique records. The search retrieved 469 records from EMBASE, 171 records from PubMed, 159 from Scopus, 169 results from Cochrane, and four results from the reference list of included studies. All articles were imported into Endnote 20 (Clarivate Analytics) for screening after removing 327 duplicate records. After the first round, 514 studies were removed by reviewing the title and abstracts. In the second round, 31 abstracts were selected for a final evaluation, which was incorporated into the analysis. A full-text review was then performed, and a total of 10 full-text articles were excluded based on the inclusion and exclusion criteria and for multiple reasons, including an unclear number of ERCPs or endpoints, Nationwide Inpatient Sample (NIS) data, review, systemic review, and meta-analysis. Finally, 21 studies met the inclusion criteria and were included in this analysis. A PRISMA flowchart illustrates our selection process, as shown in Figure 1.
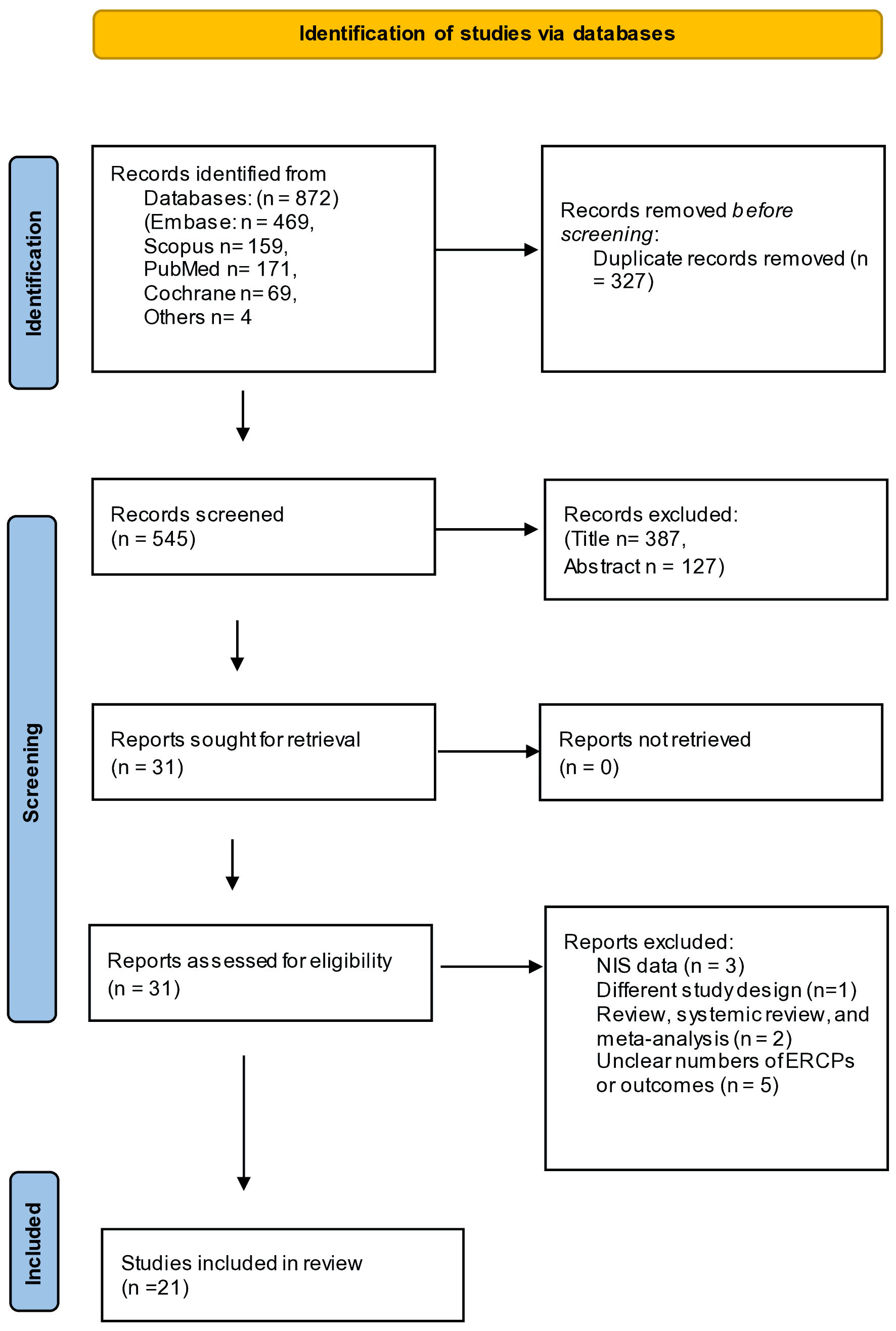 Click for large image | Figure 1. PRISMA flowchart for selection process. PRISMA: preferred reporting items for systematic reviews and meta-analyses; NIS: Nationwide Inpatient Sample. |
Metanalysis results
A total of 21 studies (Table 1) [4, 9-16, 19-30] reporting post-ERCP complications in patients with cirrhosis were included in the meta-analysis. A total of 2,576 cirrhotic patients who underwent 3,729 ERCPs were analyzed. The mean age of the patients from the included studies was 57.2 years, and the proportion of women was 34.8%. The most common cause of cirrhosis was viral hepatitis (35.7%). Most studies (16 studies) were single-center. Nine studies were conducted in Asia and eight studies in North America. A summary of the included study characteristics is presented in Table 1 [4, 9-16, 19-30]. A summary of rates of post-ERCP complications is included in Table 2.
 Click to view | Table 1. Characteristics of the Studies Included in the Meta-Analysis |
 Click to view | Table 2. Summary of Post-ERCP Complications Rates |
Post-ERCP overall complications in cirrhosis patients
To examine overall complications, 21 studies that met our inclusion criteria were included in this analysis. The pooled overall complication rate in patients with cirrhosis was 16.98% (95% CI: 13.06-21.29%, P < 0.001, I2 = 86.55%) (Fig. 2). In addition, a subgroup analysis based on the continents of the included studies was performed and showed that ERCPs performed in Asia had the highest complication rate with an overall pooled complication rate of 19.90% (95% CI: 12.43-28.56%, P < 0.001, I2 = 90.39%). On the other hand, the pooled rate of all complications was lowest in North America at 13.04% (95% CI: 8.97-17.70%, P < 0.001, I2 = 76.27%) (Fig. 2).
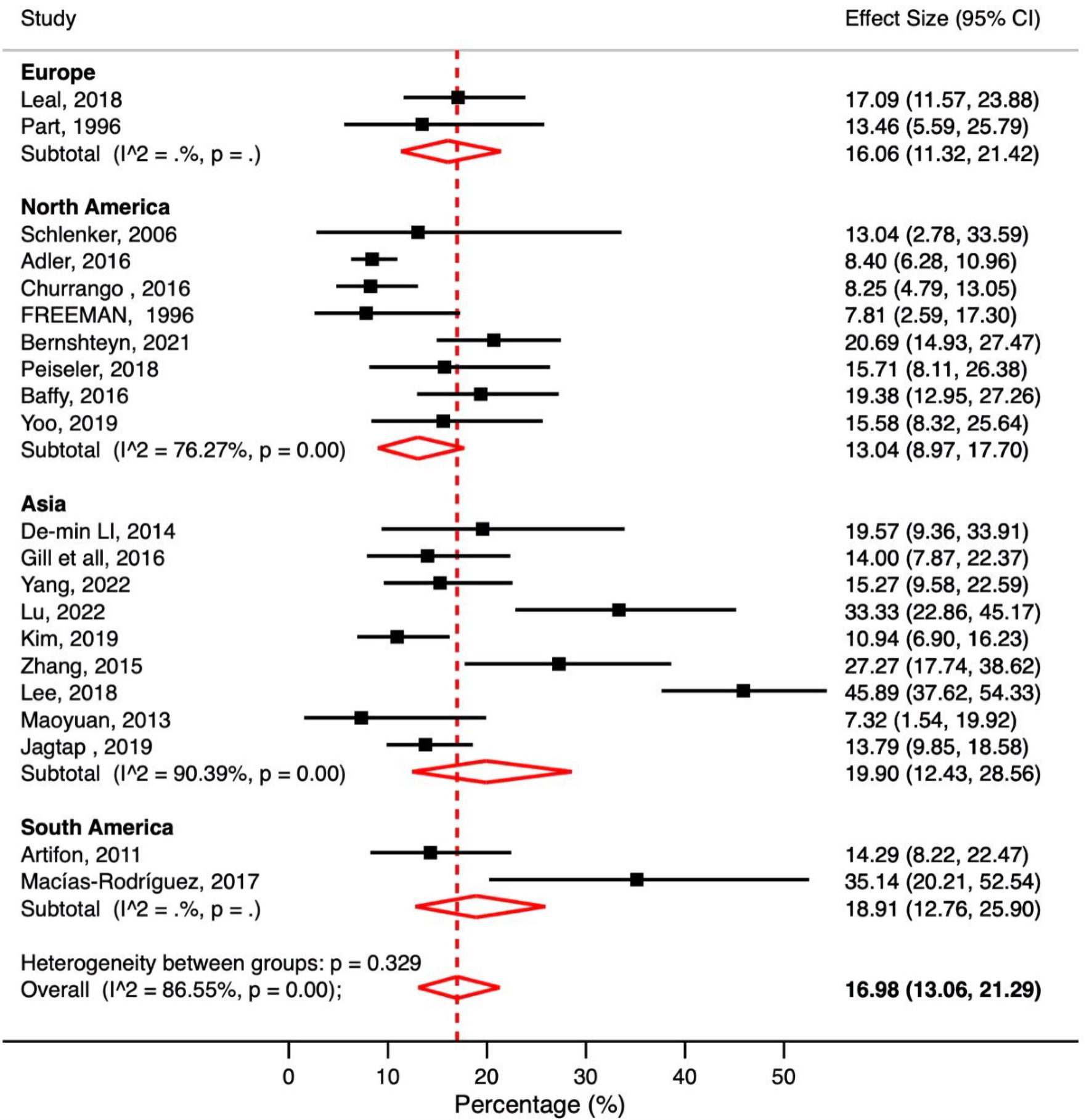 Click for large image | Figure 2. Meta-analysis of included studies reporting overall post-ERCP complications in patients with liver cirrhosis. ERCP: endoscopic retrograde cholangiopancreatography; CI: confidence interval. |
Post-ERCP bleeding
A total of 21 studies reported post-ERCP bleeding in patients with cirrhosis. The pooled post-ERCP bleeding rate in patients with cirrhosis was 5.10% (95% CI: 3.33-7.19%, P < 0.001, I2 = 76.79%) (Fig. 3).
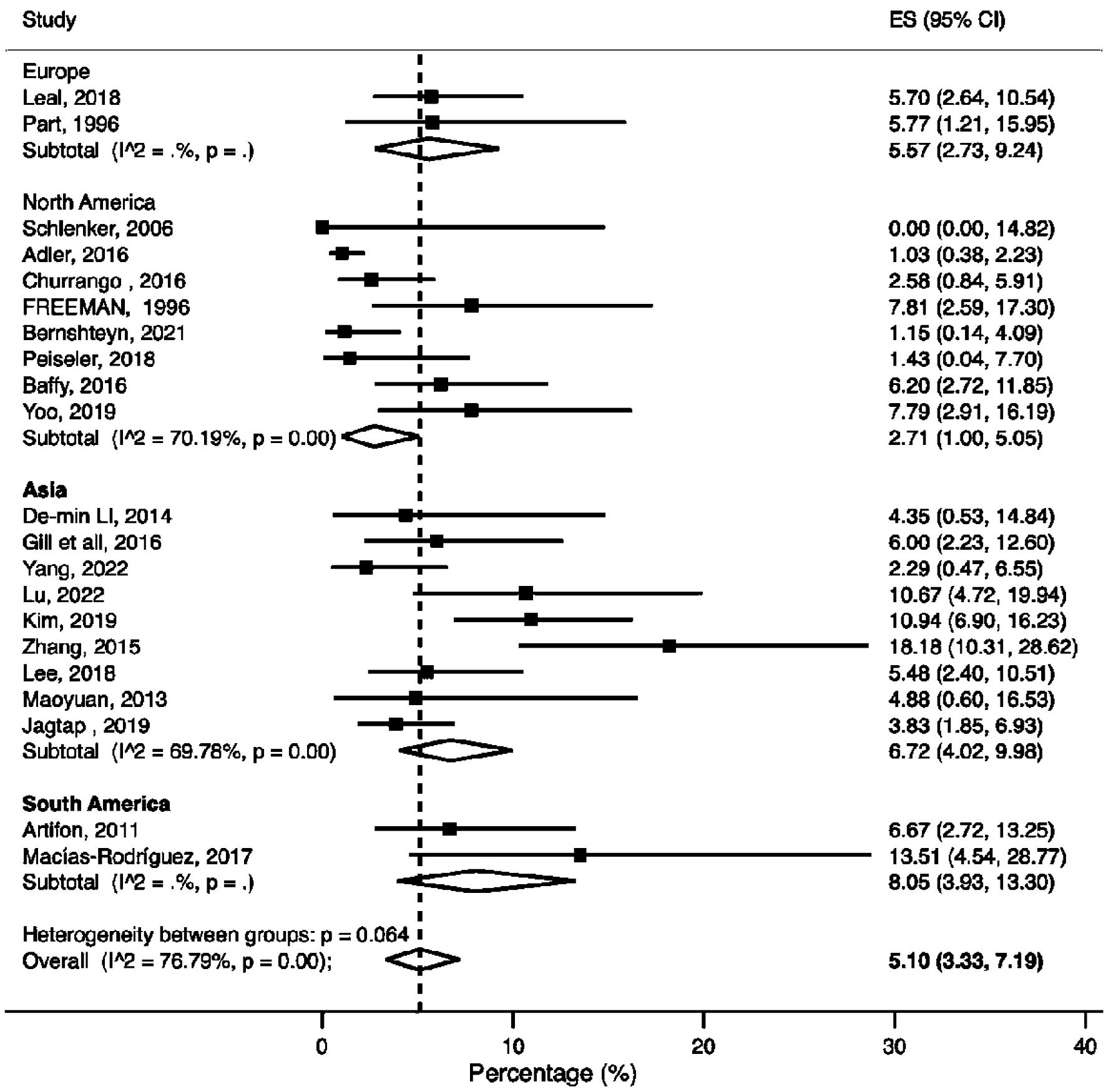 Click for large image | Figure 3. Meta-analysis of included studies reporting bleeding rate in patients with liver cirrhosis undergoing ERCP. ERCP: endoscopic retrograde cholangiopancreatography; CI: confidence interval; ES: effect size. |
PEP
A total of 19 studies reported PEP in cirrhosis patients. The pooled PEP rate in cirrhosis patients was 3.21% (95% CI: 2.20-5.36%, P = 0.03, I2 = 42.25%) (Fig. 4).
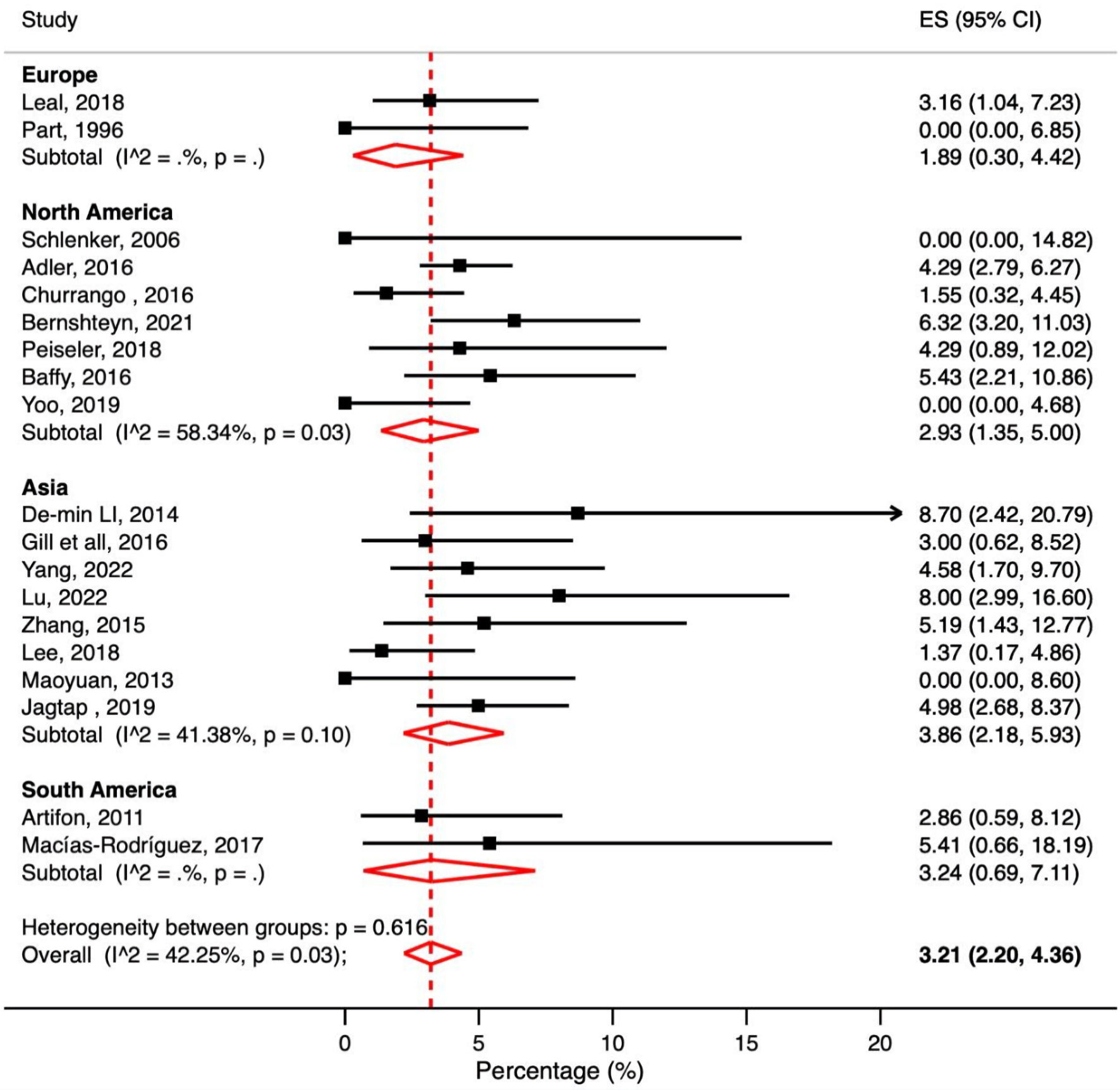 Click for large image | Figure 4. Meta-analysis of included studies reporting post-ERCP pancreatitis (PEP) rate in patients with liver cirrhosis. ERCP: endoscopic retrograde cholangiopancreatography; CI: confidence interval; ES: effect size. |
Post-ERCP cholangitis
A total of 18 studies reported post-ERCP cholangitis in patients with cirrhosis. The pooled post-ERCP cholangitis rate in patients with cirrhosis was 3.02% (95% CI: 1.19-5.52%, P < 0.001, I2 = 87.11%) (Fig. 5).
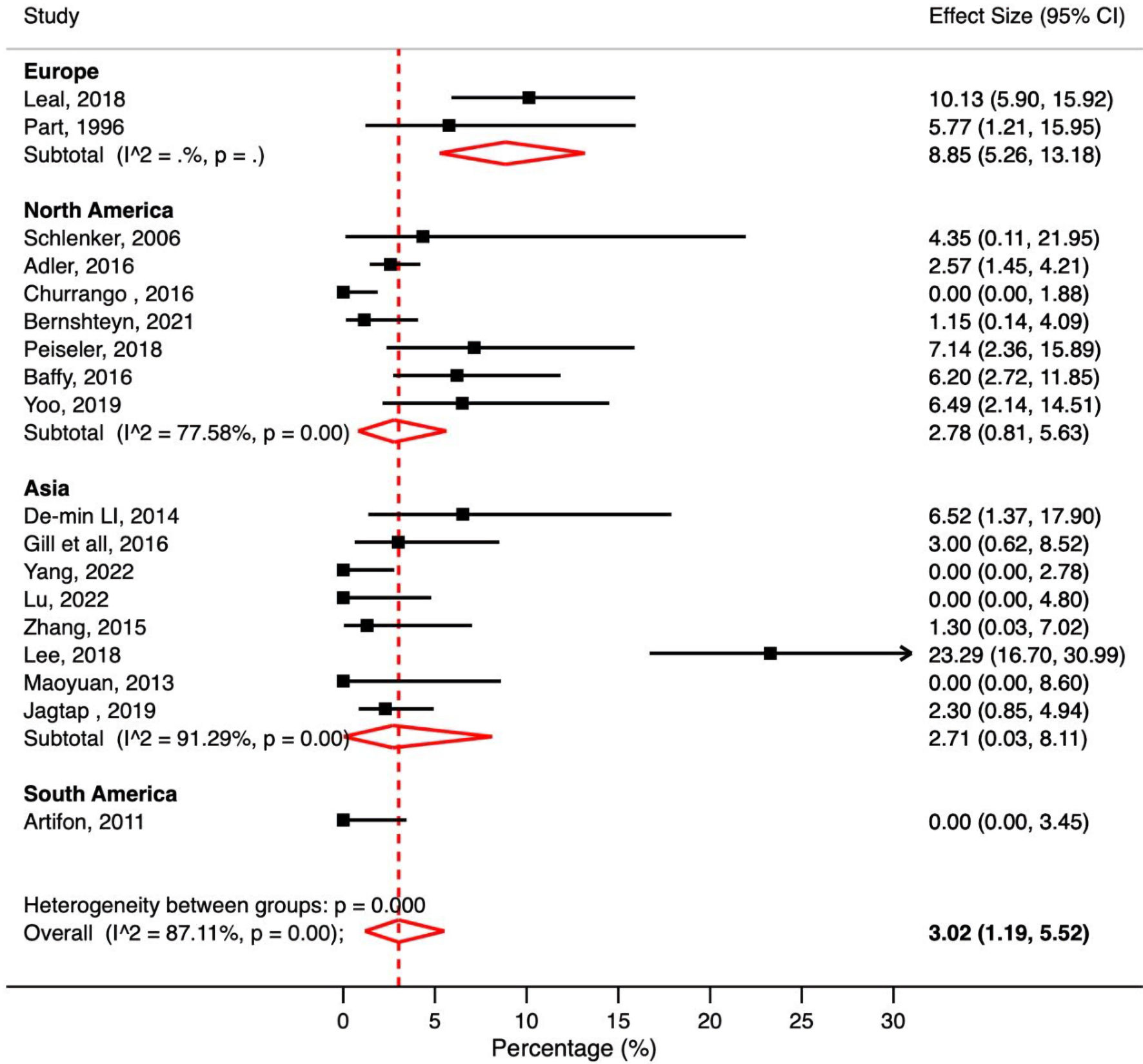 Click for large image | Figure 5. Meta-analysis of included studies reporting cholangitis rate in patient with liver cirrhosis undergoing ERCP. ERCP: endoscopic retrograde cholangiopancreatography; CI: confidence interval. |
Post-ERCP perforation
A total of 19 studies reported post-ERCP perforations in patients with cirrhosis. The pooled post-ERCP perforation rate in patients with cirrhosis was 0.12% (95% CI: 0.00-0.45%, P = 0.26, I2 = 15.76%) (Fig. 6).
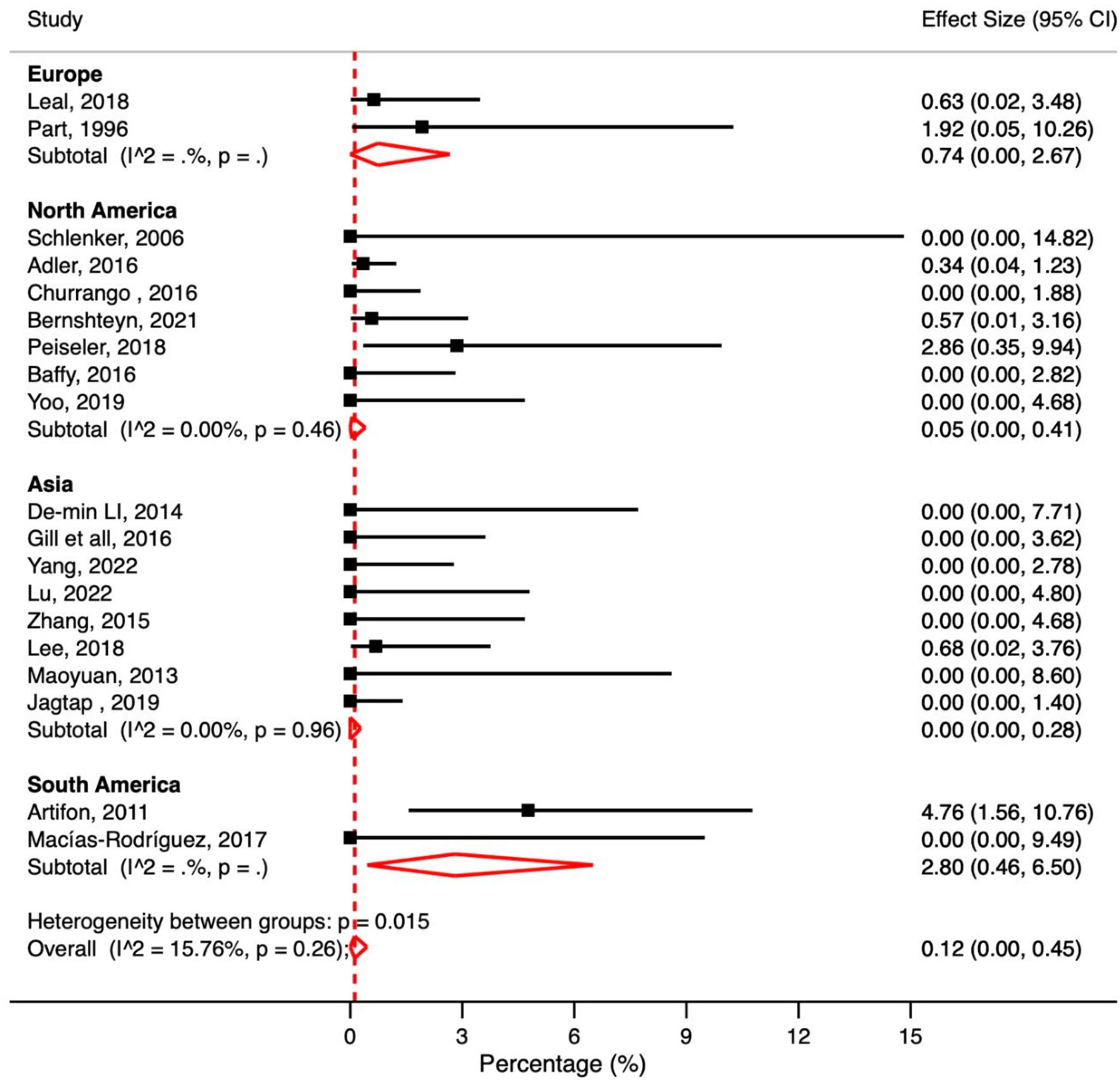 Click for large image | Figure 6. Meta-analysis of included studies reporting perforation rate in patient with liver cirrhosis undergoing ERCP. ERCP: endoscopic retrograde cholangiopancreatography; CI: confidence interval. |
Post-ERCP mortality
A total of 16 studies reported post-ERCP mortality in patients with cirrhosis. The pooled post-ERCP mortality rate in patients with cirrhosis was 0.22% (95% CI: 0.00-0.85%, P = 0.01, I2 = 51.86%) (Fig. 7).
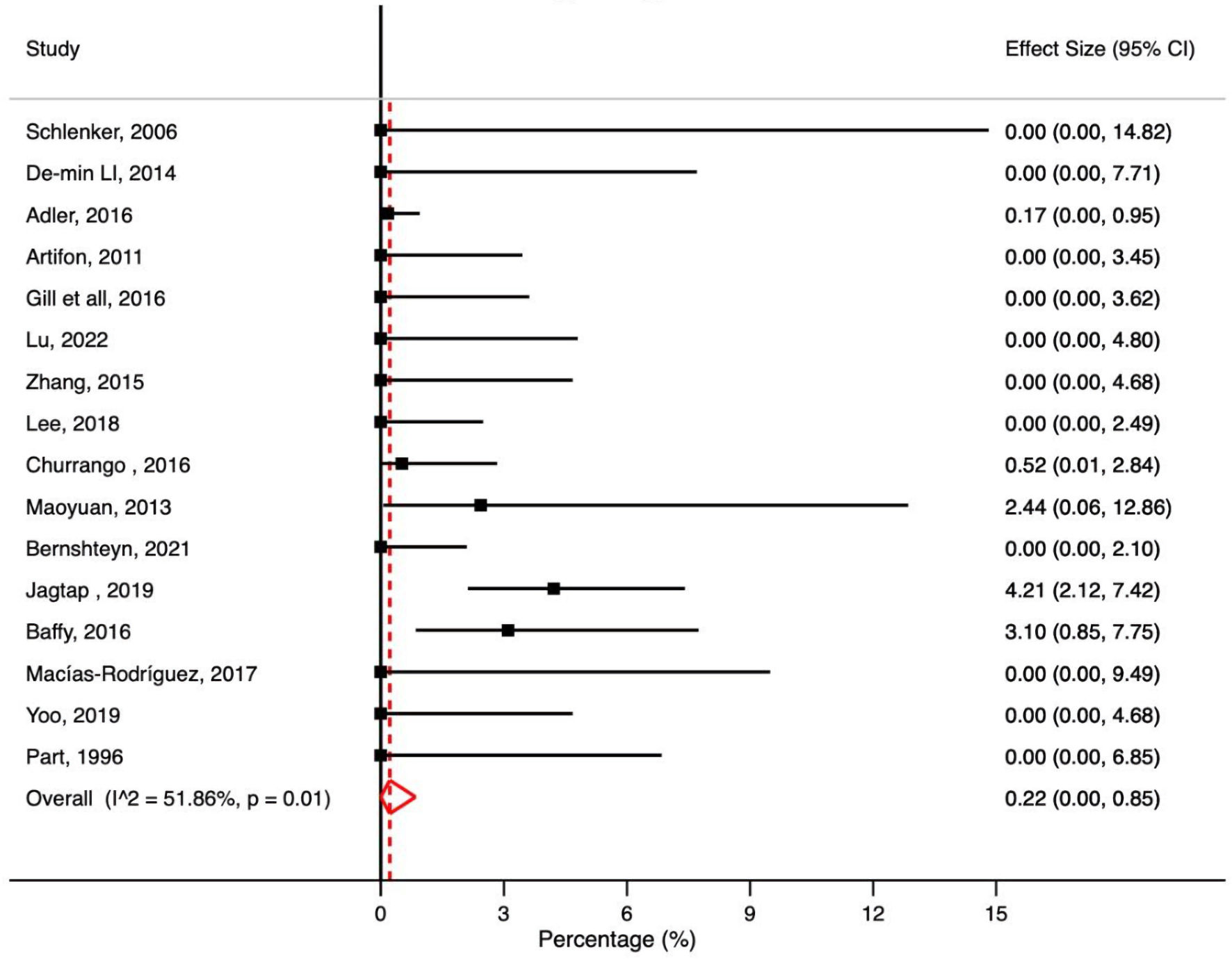 Click for large image | Figure 7. Meta-analysis of included studies reporting mortality rate in patient with liver cirrhosis undergoing ERCP. ERCP: endoscopic retrograde cholangiopancreatography; CI: confidence interval. |
Quality assessment and evaluation for publication bias
The NOS tool was used to assess the methodological quality of the observational studies included in our meta-analysis: zero studies were rated as poor quality, nine studies as moderate quality, and the remaining nine studies as high quality (Supplementary Material 1, www.gastrores.org). The Egger’s regression test and funnel charts were generated for the studies comparing complications in patients with cirrhosis and patients without cirrhosis. The plot is symmetrical and does not indicate any possible publication bias (Fig. 8).
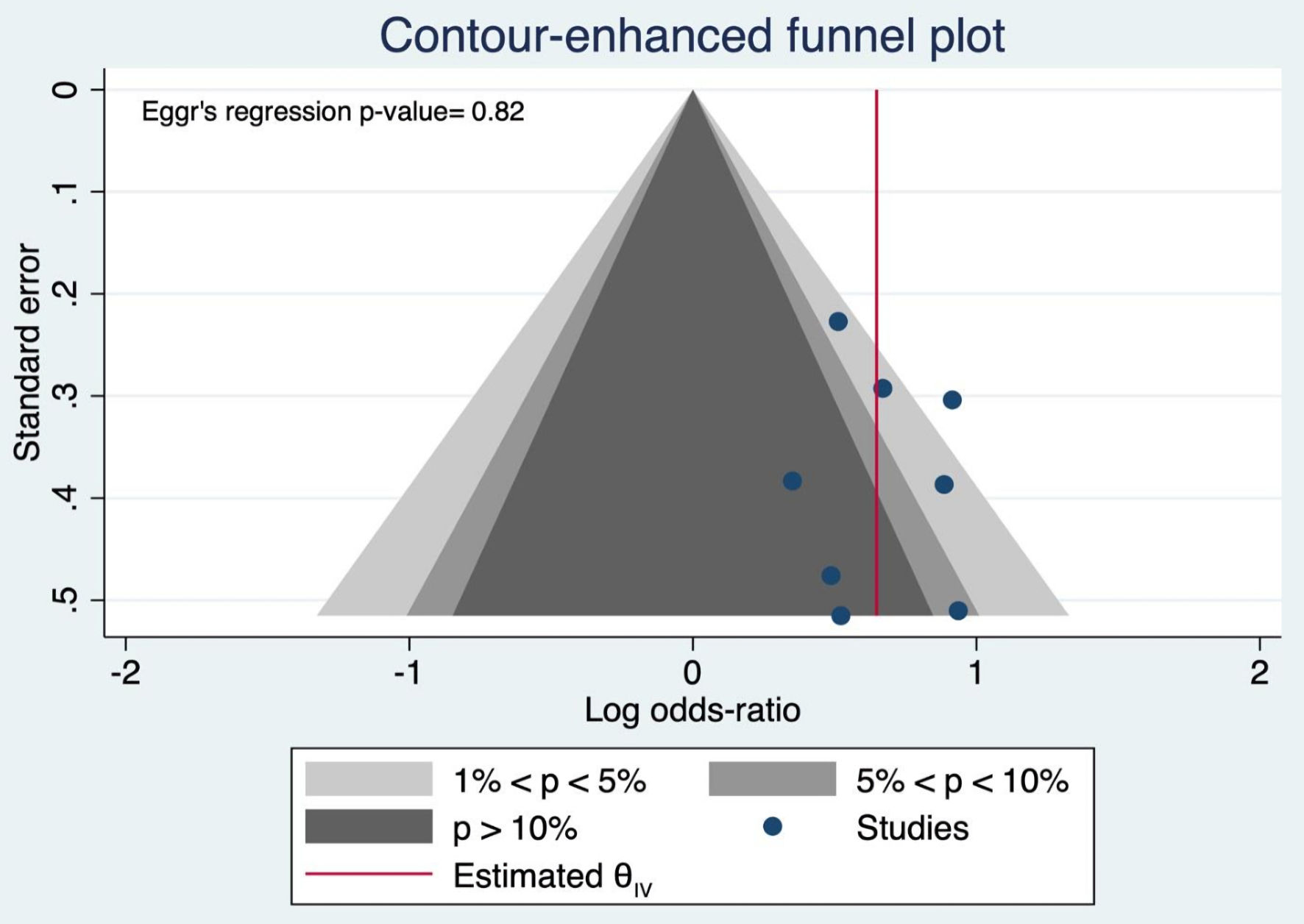 Click for large image | Figure 8. Funnel plot and Egger’s regression test. |
| Discussion | ▴Top |
In the present study, we systemically assessed post-ERCP complication rates in cirrhotic patients and analyzed intercontinental variations. We found that overall complications, post-ERCP bleeding, pancreatitis, and cholangitis are significantly high in patients with cirrhosis. We also found that ERCP performed in Asia had the highest overall post-ERCP complication rates, while North America had more favorable outcomes. In addition, studies conducted in Asia showed significantly higher post-ERCP hemorrhage and pancreatitis, while perforation rates were comparable across continents. Interestingly, higher rates of cholangitis have been found in Europe and North America. Of note, the individual post-ERCP complications do not add up to the overall complication rate due to the variability of the study sizes included in each calculation.
In this study, we observed that bleeding was the most frequently associated post-ERCP complication in patients with cirrhosis. The pooled bleeding rate was 5.1%, which is comparable to previous meta-analyses [7] showing a rate of 4.58% in patients with cirrhosis. Adler et al [22] found that bleeding was noticed in 1.1% of 328 patients with cirrhosis after ERCP. Jagtap et al [11] reported bleeding of 4.5% in 261 cirrhotic patients after ERCP for biliary indications. This correlates with the fact that patients with liver cirrhosis have an increased risk of bleeding due to derangement in coagulation factors [31]. Endoscopic sphincterotomy (EST) was found to increase the risk of developing post-ERCP bleeding in patients with cirrhosis compared to patients without cirrhosis (9.4% vs. 3.4%; P = 0.03) [15]. A multicenter retrospective study by Leal et al [15] found that the bleeding rate after ERCP was 5.7%, and a subgroup analysis showed that the presence of sphincterotomy in cirrhotic patients can further increase the bleeding risk to 9.7%, which was statistically significant. Another study by Freeman et al [19] showed that 85% of ERCP-related bleeding occurred after sphincterotomy. In addition, Child-Pugh score (CPS) class and MELD scores have been associated with the risk of developing post-ERCP bleeding. Yoo et al [12] conducted a retrospective, single-center study and found that people with a higher Child-Pugh class experienced more adverse events, including bleeding. Similarly, Li et al found that patients with Child-Pugh class C had a significantly higher risk of bleeding than patients without cirrhosis (25% vs. 3%) [20]. Zhang et al [4] showed that patients with ERCP-related bleeding had MELD scores of 11.5 or higher, while Macias-Rodriguez et al [21] suggested that a MELD score of 16 or higher had a higher risk of bleeding.
This meta-analysis showed that the pooled PEP rate in patients with cirrhosis was 3.21%. This is similar to the previous meta-analysis [7], which showed a PEP rate of 3.58% in patients with cirrhosis. Adler et al [22] reported that 4.6% of 328 patients with cirrhosis developed PEP, while Jagtap et al [11] reported PEP of 5.8% in a cohort of 261 cirrhotic patients after ERCP for biliary indications. The risks of PEP are multifactorial, and EST has been associated with a higher risk of PEP. Adler et al [22] performed a subgroup analysis in patients with liver cirrhosis and found that PEP rate was significantly higher in patients with sphincterotomy than in those without (12.8 vs. 2.5%). Zhang et al [4] found that PEP was more common in cirrhotic patients undergoing endoscopic papillary balloon dilatation than EST (16% vs. 0%). This also agrees with Lee et al [32], who reported that PEP occurred at a rate of 5.8% in patients with decompensated cirrhosis compared to 0.0% in compensated cirrhosis. Furthermore, a multivariate analysis by Kim et al [13] showed that stent placement in patients with cirrhosis was associated with a higher likelihood of developing PEP. It is important to note that the endoscopist’s experience and the technique of ERCP play a crucial role in the development of pancreatitis [32, 33]. In a study by Li et al [20], the incidence of PEP was similar in cirrhotic and non-cirrhotic patients. They found that the rates of bile duct access and bile duct clearance were comparable between these two groups. This could be explained by the fact that the same endoscopist performed all interventions. Ma et al [23] conducted a retrospective single-center study with 41 patients in China where PEP did not occur in any patient. This could be due to the smaller number of patients involved, endoscopists’ experience, or the ERCP technique.
The pooled post-ERCP cholangitis rate in patients with cirrhosis was 3.02% compared to 1.93% in a previous meta-analysis [7]. In the study by Leal et al [15], post-ERCP cholangitis rates were higher in patients with cirrhosis than in those without cirrhosis. However, the difference in rates between the two groups was not significant when analyzed for the presence of a sphincterotomy. Lee et al [32] found statistically significantly higher rates of post-ERCP cholangitis in patients with decompensated cirrhosis compared to patients with compensated cirrhosis. Kim et al [13] found that stent placement was associated with a higher likelihood of post-ERCP cholangitis. This is further supported by Peiseler et al [14], who concluded that temporary stent placement was identified as a significant risk factor for post-ERCP cholangitis.
We analyzed 16 studies reporting post-ERCP mortality in patients with cirrhosis. The pooled post-ERCP mortality rate in patients with cirrhosis was 0.22%. Jagtap et al [11] reported that cholangitis significantly increases the risk of mortality in patients with decompensated liver cirrhosis undergoing ERCP. A national inpatient sample database study [2] found that in-hospital ERCP-related mortality was higher in patients with cirrhosis compared to those without cirrhosis, with age being associated with higher mortality rates. The same study [2] also showed a higher mortality risk in patients who developed ERCP-associated complications than those without complications.
We analyzed the intercontinental variation in post-ERCP complications in cirrhotic patients. We found that overall complications, post-ERCP bleeding, and PEP were higher in studies conducted in Asia and South America. Regarding PEP, the risks are multifactorial, but as we have already mentioned, the endoscopist’s experience and the ERCP technique play a central role in PEP [32, 33]. Interestingly, cholangitis rates were significantly higher in Europe and North America. These results can be attributed partly to heterogeneity in cross-continent practices among physicians performing ERCP across continents. Compared to more commonly performed standard gastrointestinal procedures such as esophagogastroduodenoscopy and colonoscopy, ERCP is far more difficult to perform, requires greater physician skill, and a longer learning curve to gain advanced proficiency [34]. An appropriate level of backup infrastructure and technical expertise should be available to ensure the security of the procedures. These features may contribute to differences in ERCP adverse events in different hospital settings across countries [34]. A recent study by Verma et al [33] showed that at least 350 native papillae cannulations are required to achieve an 80% probability of successful deep bile duct cannulation. Achieving this number during the fellowship period requires a solid training program with a large caseload for adequate exposure. This requires rigorous training and a steep learning curve for the endoscopist. The American Society for Gastrointestinal Endoscopy proposed several recommendations for quality indicators during and after the procedure [35, 36]. Strict adherence to these recommendations is critical. However, it should be considered that maintaining the quality indicator may not be achievable even in developed countries, as reported in a prospective survey involving five regions of the UK [37].
The limitations of this study include the high heterogeneity of the included studies. This could limit the generalizability of the results of this study. Also, not all of the included studies clearly identified the indications for ERCP in patients with liver cirrhosis. Other limitations include variability in the severity of cirrhosis, its etiology, and the severity of the adverse events relative to the severity of the cirrhosis. Also, there was variability in methods for diagnosing liver cirrhosis (clinical diagnosis, biopsy, or imaging). We also could not obtain cardiopulmonary or anesthesia adverse events in the analysis due to the lack of these adverse events in most studies. In addition, the performance of ERCP is influenced by the institution’s capabilities, patient load, and the physician’s experience. Finally, the retrospective designs of these studies may serve as a limitation as the procedures were not standardized, being performed at different sites and by different endoscopists. Despite these limitations, the strength of this meta-analysis is its pooling of 21 studies and large sample sizes, including 2,576 cirrhotic patients. In addition to analyzing post-ERCP complications, we analyzed mortality rates, which add a crucial element when considering ERCP. We also analyzed cross-continent differences in ERCP complications, underscoring the urgent need for national collaborations.
In conclusion, our systematic review and meta-analysis found that overall complications, post-ERCP bleeding, pancreatitis, and cholangitis are significantly more common in patients with cirrhosis. In addition, we also showed intercontinental differences in these complications. Because cirrhotic patients are more likely to experience post-ERCP complications, a comprehensive decision-making process considering the benefit-risk balance is required. More importantly, it is time for policymakers and national societies in different countries to share ERCP experiences to optimize post-ERCP complications in this complex cohort.
| Supplementary Material | ▴Top |
Suppl 1. Quality assessment of the included studies using the NOS.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
All authors have no conflict of interest to disclose.
Informed Consent
Patient consent was not required as the meta-analysis studies did not include human subjects.
Author Contributions
Conception and design: S. Alsakarneh, F. Jaber, HM Ghoz, JH Helzberg. Figures and table: K. Ahmed, W. Mohammad, T. Bierman. Provision of study materials or patients: S. Alsakarneh, F. Jaber, HM Ghoz, JH Helzberg, M. ShahMiran, J. Campbell. Collection and assembly of data: WT Mohamed, MK Ahmed, Y Abboud, MK Almujarkesh. Data analysis and interpretation: F. Ghanem. Manuscript writing: All authors. Final approval of manuscript: all authors.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
ERCP: endoscopic retrograde cholangiopancreatography; CI: confidence interval; RR: risk ratio: ; OR: odds ratio; PEP: post-ERCP pancreatitis
| References | ▴Top |
- Kroner PT, Bilal M, Samuel R, Umar S, Abougergi MS, Lukens FJ, Raimondo M, et al. Use of ERCP in the United States over the past decade. Endosc Int Open. 2020;8(6):E761-E769.
doi pubmed pmc - Solanki S, Kichloo A, Dahiya DS, Solanki D, Singh J, Wani F, Albosta M, et al. Endoscopic Retrograde Cholangiopancreatography (ERCP) in patients with liver cirrhosis: analysis of trends and outcomes from the national inpatient sample database. J Clin Gastroenterol. 2022;56(7):618-626.
doi pubmed pmc - Ahmed M, Kanotra R, Savani GT, Kotadiya F, Patel N, Tareen S, Fasullo MJ, et al. Utilization trends in inpatient endoscopic retrograde cholangiopancreatography (ERCP): A cross-sectional US experience. Endosc Int Open. 2017;5(4):E261-E271.
doi pubmed pmc - Zhang J, Ye L, Zhang J, Lin M, He S, Mao X, Zhou X, et al. MELD scores and Child-Pugh classifications predict the outcomes of ERCP in cirrhotic patients with choledocholithiasis: a retrospective cohort study. Medicine (Baltimore). 2015;94(3):e433.
doi pubmed pmc - Acalovschi M, Badea R, Dumitrascu D, Varga C. Prevalence of gallstones in liver cirrhosis: a sonographic survey. Am J Gastroenterol. 1988;83(9):954-956.
pubmed - Alkhatib AA, Hilden K, Adler DG. Comorbidities, sphincterotomy, and balloon dilation predict post-ERCP adverse events in PSC patients: operator experience is protective. Dig Dis Sci. 2011;56(12):3685-3688.
doi pubmed - Mashiana HS, Dhaliwal AS, Sayles H, Dhindsa B, Yoo JW, Wu Q, Singh S, et al. Endoscopic retrograde cholangiopancreatography in cirrhosis - a systematic review and meta-analysis focused on adverse events. World J Gastrointest Endosc. 2018;10(11):354-366.
doi pubmed pmc - Navaneethan U, Njei B, Zhu X, Kommaraju K, Parsi MA, Varadarajulu S. Safety of ERCP in patients with liver cirrhosis: a national database study. Endosc Int Open. 2017;5(4):E303-E314.
doi pubmed pmc - Yang H, Mou Y, Hu B. Safety and efficacy of common endoscopic treatments in patients with decompensated liver cirrhosis. Ann Hepatol. 2022;27(3):100689.
doi pubmed - Bernshteyn M, Hu L, Masood U, Sharma AV, Huang D, Sapkota B. Retrospective analysis of complications related to endoscopic retrograde cholangio-pancreatography in patients with cirrhosis vs patients without cirrhosis. World J Hepatol. 2021;13(4):472-482.
doi pubmed pmc - Jagtap N, Nabi Z, Tandan M, Ramchandani M, Sharma M, Lakhtakia S, Rao PN, et al. Is it safe to perform endoscopic retrograde cholangiopancreatography in decompensated cirrhosis? J Clin Exp Hepatol. 2019;9(5):554-560.
doi pubmed pmc - Yoo T, Epistola R, Epistola J, Ku L, Fleischman MW, Reicher S, Eysselein VE, et al. Evaluating the risk of adverse events with interventional endoscopic retrograde cholangiopancreatography and endoscopic ultrasound procedures in cirrhotic patients. World J Gastrointest Endosc. 2019;11(11):523-530.
doi pubmed pmc - Kim JY, Lee HS, Chung MJ, Park JY, Park SW, Song SY, Bang S. Bleeding complications and clinical safety of endoscopic retrograde cholangiopancreatography in patients with liver cirrhosis. Yonsei Med J. 2019;60(5):440-445.
doi pubmed pmc - Peiseler M, Reiners D, Pinnschmidt HO, Sebode M, Jung F, Hartl J, Zenouzi R, et al. Risk of endoscopic biliary interventions in primary sclerosing cholangitis is similar between patients with and without cirrhosis. PLoS One. 2018;13(8):e0202686.
doi pubmed pmc - Leal C, Prado V, Colan J, Chavez-Rivera K, Sendino O, Blasi A, Roura P, et al. Adverse Events and Acute Chronic Liver Failure in Patients With Cirrhosis Undergoing Endoscopic Retrograde Cholangiopancreatography: A Multicenter Matched-Cohort Study. Am J Gastroenterol. 2019;114(1):89-97.
doi pubmed - Lu HS, Yang TC, Chang CY, Huang YH, Hou MC. The risk of variceal bleeding during endoscopic retrograde cholangiopancreatography. J Chin Med Assoc. 2022;85(9):896-900.
doi pubmed - Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336-341.
doi pubmed - Wells GA, Wells G, Shea B, Shea B, O'Connell D, Peterson J, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2014.
- Freeman ML, Nelson DB, Sherman S, Haber GB, Herman ME, Dorsher PJ, Moore JP, et al. Complications of endoscopic biliary sphincterotomy. N Engl J Med. 1996;335(13):909-918.
doi pubmed - Li DM, Zhao J, Zhao Q, Qin H, Wang B, Li RX, Zhang M, et al. Safety and efficacy of endoscopic retrograde cholangiopancreatography for common bile duct stones in liver cirrhotic patients. J Huazhong Univ Sci Technolog Med Sci. 2014;34(4):612-615.
doi pubmed - Macias-Rodriguez RU, Ruiz-Margain A, Rodriguez-Garcia JL, Zepeda-Gomez S, Torre A. Risk factors associated with complications in cirrhotic patients undergoing endoscopic retrograde cholangio-pancreatography. Eur J Gastroenterol Hepatol. 2017;29(2):238-243.
doi pubmed - Adler DG, Haseeb A, Francis G, Kistler CA, Kaplan J, Ghumman SS, Laique SN, et al. Efficacy and safety of therapeutic ERCP in patients with cirrhosis: a large multicenter study. Gastrointest Endosc. 2016;83(2):353-359.
doi pubmed - Ma MY JG, Wang X, Mu L, Ji GZ, Wang M. ERCP for treatment of choledocholithiasis in patients with liver cirrhosis. World Chinese Journal of Digestology. 2013.
- Lee JC, Kim JS, Kim HW, Cho IK, Lee J, Jang ES, Lee SH, et al. Outcome of endoscopic retrograde cholangiopancreatography in patients with clinically defined decompensated liver cirrhosis. J Dig Dis. 2018;19(10):605-613.
doi pubmed - Baffy N, Adike A, Al-Qaisi M, et al. Risk of adverse events following ERCP in patients with cirrhosis: 925. Official journal of the American College of Gastroenterology | ACG. 2016;111.
- Gill M, Yousuf SGN, Nawaz A. Outcomes of endoscopic retrograde cholangiopancreatography in patients with cirrhosis. Journal of Hepatology. 2016.
- Churrango J, Doppalapudi K, Hsieh A, Ahlawat S. Sa1171 predictors of post-ERCP complications in patients with liver cirrhosis. Gastrointestinal Endoscopy. 2016;83(5):AB240-AB1
- Artifon EL, da Silveira EB, Aparicio D, Takada J, Baracat R, Sakai CM, Garcia RT, et al. Management of common bile duct stones in cirrhotic patients with coagulopathy: a comparison of supra-papillary puncture and standard cannulation technique. Dig Dis Sci. 2011;56(6):1904-1911.
doi pubmed - Schlenker C, Trotter JF, Shah RJ, Everson G, Chen YK, Antillon D, Antillon MR. Endoscopic gallbladder stent placement for treatment of symptomatic cholelithiasis in patients with end-stage liver disease. Am J Gastroenterol. 2006;101(2):278-283.
doi pubmed - Prat F, Tennenbaum R, Ponsot P, Altman C, Pelletier G, Fritsch J, Choury AD, et al. Endoscopic sphincterotomy in patients with liver cirrhosis. Gastrointest Endosc. 1996;43(2 Pt 1):127-131.
doi pubmed - Flores B, Trivedi HD, Robson SC, Bonder A. Hemostasis, bleeding and thrombosis in liver disease. J Transl Sci. 2017;3(3).
doi pubmed pmc - Lee HJ, Cho CM, Heo J, Jung MK, Kim TN, Kim KH, Kim H, et al. Impact of hospital volume and the experience of endoscopist on adverse events related to endoscopic retrograde cholangiopancreatography: a prospective observational study. Gut Liver. 2020;14(2):257-264.
doi pubmed pmc - Verma D, Gostout CJ, Petersen BT, Levy MJ, Baron TH, Adler DG. Establishing a true assessment of endoscopic competence in ERCP during training and beyond: a single-operator learning curve for deep biliary cannulation in patients with native papillary anatomy. Gastrointest Endosc. 2007;65(3):394-400.
doi pubmed - Talukdar R, Nageshwar Reddy D. ERCP in developing countries: the way forward. Gastrointest Endosc. 2016;84(4):667-669.
doi pubmed - Adler DG, Lieb JG, 2nd, Cohen J, Pike IM, Park WG, Rizk MK, Sawhney MS, et al. Quality indicators for ERCP. Gastrointest Endosc. 2015;81(1):54-66.
doi pubmed - Baron TH, Petersen BT, Mergener K, Chak A, Cohen J, Deal SE, Hoffman B, et al. Quality indicators for endoscopic retrograde cholangiopancreatography. Gastrointest Endosc. 2006;63(4 Suppl):S29-S34.
doi pubmed - Williams EJ, Taylor S, Fairclough P, Hamlyn A, Logan RF, Martin D, Riley SA, et al. Are we meeting the standards set for endoscopy? Results of a large-scale prospective survey of endoscopic retrograde cholangio-pancreatograph practice. Gut. 2007;56(6):821-829.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


