| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Case Report
Volume 16, Number 2, April 2023, pages 118-124
Esophageal Ulcer After Intravitreal Ranibizumab Injection in a Patient With Age-Related Macular Degeneration
Xin Qing Lia, d, e, Ke Wei Zhub, d , Jun Laia, Jian Wua, Xiao Fang Guoc
aDepartment of Pharmacy, Ganzhou People’s hospital (The Affiliated Ganzhou Hospital of Nanchang University), Ganzhou 341000, Jiangxi Province, China
bOffice of Pharmacovigilance, Guangzhou Baiyunshan Pharmaceutical Holding Co., Ltd. Baiyunshan Pharmaceutical General Factory, Guangzhou 510515, Guangdong Province, China
cDepartment of Gastroenterology, Ganzhou People’s hospital (The Affiliated Ganzhou Hospital of Nanchang University), Ganzhou 341000, Jiangxi Province, China
dThese authors have contributed equally to this article.
eCorresponding Author: Xin Qing Li, Department of Pharmacy, Ganzhou People’s hospital (The Affiliated Ganzhou Hospital of Nanchang University), Ganzhou 341000, Jiangxi Province, China
Manuscript submitted February 2, 2023, accepted March 16, 2023, published online April 28, 2023
Short title: Ranibizumab-Induced Esophageal Ulcer
doi: https://doi.org/10.14740/gr1603
| Abstract | ▴Top |
Ranibizumab is a monoclonal antibody fragment targeted against vascular endothelial growth factor (VEGF) A isoform (VEGF-A). This study aimed to report a case of esophageal ulcer that developed soon after intravitreal ranibizumab injection in a patient with age-related macular degeneration (AMD). A 53-year-old male patient diagnosed with AMD received ranibizumab through intravitreal injection in the left eye. Mild dysphagia occurred 3 days after receiving intravitreal ranibizumab injection for the second time. The dysphagia exacerbated remarkably and was accompanied by hemoptysis 1 day after receiving ranibizumab for the third time. Severe dysphagia accompanied by intense retrosternal pain and pant emerged after injecting ranibizumab for the fourth time. An esophageal ulcer was observed through ultrasound gastroscopy, covered with fibrinous tissue, and surrounded by flushing and congestive mucosae. The patient received proton pump inhibitor (PPI) therapy combined with traditional Chinese medicine (TCM) after discontinuation of ranibizumab. The dysphagia and retrosternal pain were gradually relieved after treatment. Afterwards, the esophageal ulcer has not relapsed since permanent discontinuation of ranibizumab. To our best knowledge, this was the first case of esophageal ulcer related to intravitreal ranibizumab injection. Our study indicated that VEGF-A played a potential role in the development of esophageal ulceration.
Keywords: Esophageal ulcer; Ranibizumab; Age-related macular degeneration; Vascular endothelial growth factor; Bevacizumab
| Introduction | ▴Top |
Ranibizumab, as a monoclonal antibody fragment targeted against vascular endothelial growth factor (VEGF) A isoform (VEGF-A), is used to treat age-related macular degeneration (AMD) through intravitreal injection [1]. AMD is a leading cause of irreversible blindness for old patients in developed countries [2]. VEGF-A play a key role in the progression of wet AMD, which is a major cause of severe vision loss [2]. Ranibizumab exhibits excellent effectiveness in the treatment of AMD and prevents moderate visual loss in ≥ 90% of patients [3]. The recommended dose of ranibizumab is 0.5 mg every month, which is sufficient to improve the clinical outcome in AMD patients. Meanwhile, ranibizumab is well tolerated with a low incidence of serious adverse reactions [4]. The common ocular adverse reactions of ranibizumab include conjunctival bleeding, eye pain, vitreous floaters, and increased intraocular pressure. VEGF inhibition leads to systemic adverse reactions, such as arterial thromboembolic events, hypertension, nonocular bleeding, proteinuria, urinary tract infections, and pneumonia [4, 5]. In addition, gastrointestinal disorders occur with a low incidence after intravitreal injection of ranibizumab, including bleeding, nausea, and vomiting [5]. We report a rare case of esophageal ulcer following intravitreal ranibizumab injection in a patient with AMD.
Esophageal ulcers are defined as discrete breaks in esophageal mucosae with clearly identifiable margins, with a reported prevalence of 1.2% [6]. The common symptoms of esophageal ulceration at presentation are odynophagia, retrosternal pain, and dysphagia [7].
| Case Report | ▴Top |
A 53-year-old male was admitted to the First Affiliated Hospital of Gannan Medical University (Ganzhou, Jiangxi Province, China) on September 13, 2021, with a complaint of sudden impaired vision for 8 days. The visual acuity of the right eye (VOD) was 1.0, while the visual acuity of the left eye (VOS) was 0.05. Intraocular pressures (IOPs) measured by a noncontact tonometer were 18.0 mm Hg and 17.0 mm Hg in the right eye and left eye, respectively. Massive hemorrhage and exudation accompanied by edema were observed in the left macula.
After diagnosis of AMD, the patient was intravitreally injected with ranibizumab (0.5 mg; Novartis Pharma Schweiz AG, Basel, Switzerland) following surface anesthesia in the First Affiliated Hospital of Gannan Medical University (Ganzhou, Jiangxi Province, China) on September 14, 2021. No obvious adverse reaction occurred after administration of ranibizumab. The patient received ranibizumab with a duplicate way on October 14, 2021 (VOD, 1.0; VOS, 0.05; IOP: right eye 19 mm Hg, left eye 20 mm Hg). Slight difficulty of swallowing accompanied by choking symptoms emerged during intake of food 3 days after ranibizumab injection and was alleviated without treatment in the following days. The patient received ranibizumab for the third time on November 25, 2021 (VOD, 1.0; VOS, 0.04; IOP: right eye 18 mm Hg, left eye 18 mm Hg). Dysphagia aggravated remarkably on the second day, and hemoptysis emerged with low amount of blood, which was regarded as gingival bleeding by the patient and failed to bring to the attention. The dysphagia and hemoptysis were relieved without treatment in the following days. The patient was intravitreally injected with ranibizumab 0.5 mg for the fourth time on January 6, 2022 (VOD, 1.0; VOS, 0.2; IOP: right eye 20 mm Hg, left eye 19 mm Hg). Severe dysphagia accompanied by intense retrosternal pain and pant occurred during intake of solid or liquid food on the second day. On the other hand, the hemorrhage, exudation, and edema in the left macula were dramatically alleviated. On January 13, 2022, the patient underwent an electronic gastroscopy test in the People’s Hospital of Ningdu County, Ganzhou, China. An esophageal ulcer with a size of approximately 5 × 1 cm was observed 20 - 25 cm from the incisor, covered with fibrinous tissue, and surrounded by congestive and edematous mucosae with a high risk of hemorrhage.
The hospital recommended the patient to receive therapy in the Ganzhou People’s Hospital, Ganzhou, China. The patient was admitted to the Ganzhou People’s Hospital in 5 days. An ultrasound gastroscopy test was performed after hospitalization, and the result revealed that an esophageal surface ulcer was visualized 20 - 24 cm from the incisor. The central of the ulcer was covered with fibrinous tissue, and the surrounding mucosae were flushing and congestive (Fig. 1a). Ultrasonography revealed that the mucosae surrounding the esophageal ulcer thickened slightly with a hypoechoic variation that indicated a normal state, and no other structural abnormity was detected (Fig. 1b). Lugol solution (Gram Stain, Beijing Solarbio Science & Technology Co., Ltd. Beijing, China) was sprayed to the esophageal ulcer through the magnifying endoscopy, and only the fibrinous tissue was stained as a result, indicating that there was an ulcer or tumor (Fig. 1c). No ulcer or bump was observed in the fundus, body, and angle of the stomach, as well as the cardia, pylorus, and duodenum (Fig. 1d). Magnifying endoscopy with narrow-band imaging (ME-NBI) visualized the boundary of the esophageal ulcer with color transformation to hazel, and no abnormal blood vessel was observed, indicating that no intestinal metaplasia or cancerization occurred in the esophageal ulcer (Fig. 2). Good tissue flexibility was detected using a biopsy. In addition, an esophageal biopsy was performed to confirmed that the lesion was an ulcer. As shown in Figure 3, the lesion surface was covered with squamous epithelium, and a large amount of small vascular hyperplasia with inflammatory cell infiltration was observed in the mucosa and submucosa. The epithelium was detached in some areas, and inflammatory exudative necrosis was observed. To sum up, the patient was diagnosed with an esophageal surface ulcer.
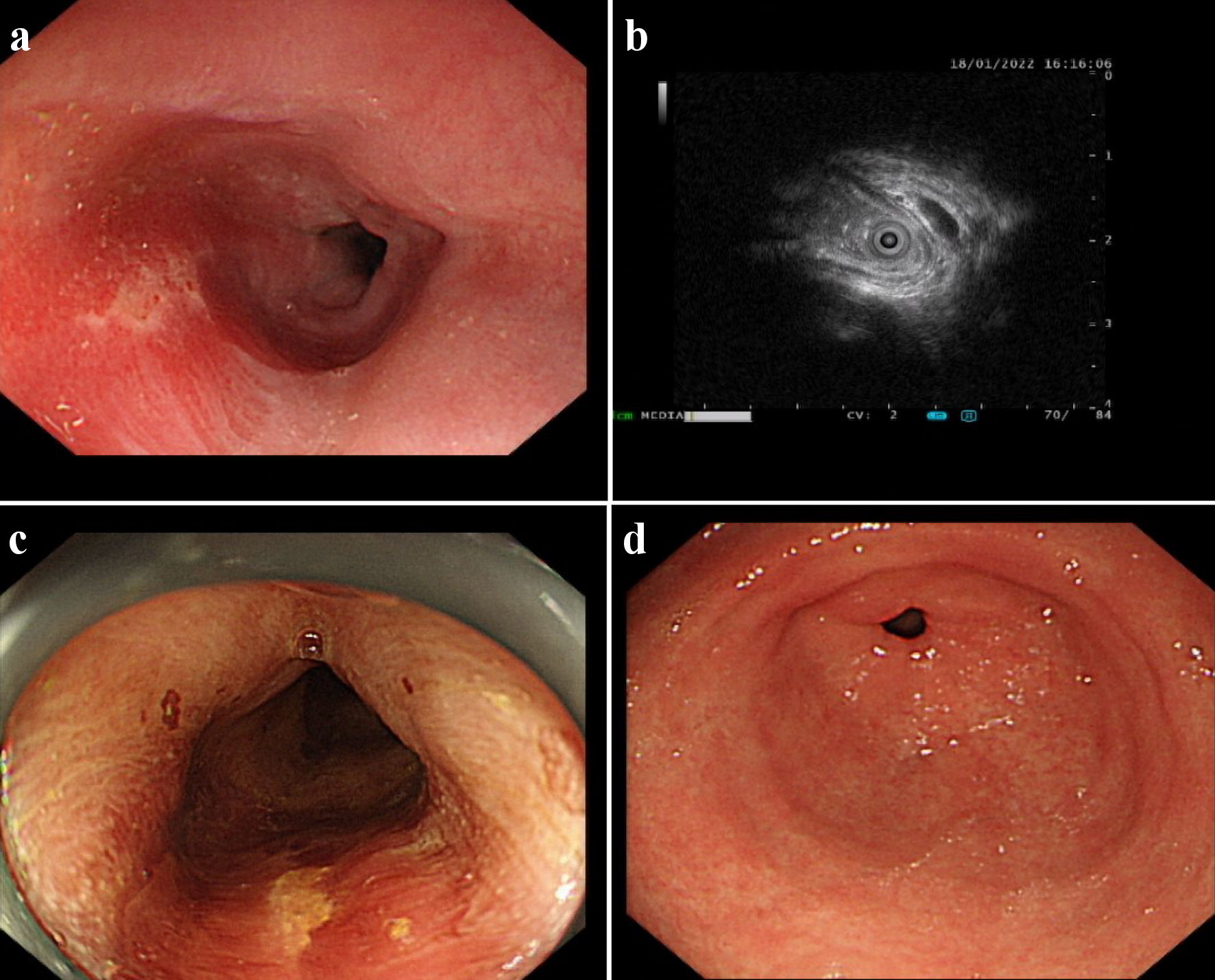 Click for large image | Figure 1. An esophageal surface ulcer occurred after intravitreal ranibizumab injection. (a) Ultrasound gastroscopy showed an esophageal surface ulcer 20 - 24 cm from the incisor. The central of ulcer was covered with fibrinous tissue, and the surrounding mucosae were flushing and congestive. (b) Ultrasonography revealed that the mucosae were slightly incrassated with a hypoechoic variation in the lesion area. (c) The fibrinous tissue was stained by Lugol solution. (d) Ultrasound gastroscopy showed that the mucosae in the gastric antrum were smooth. |
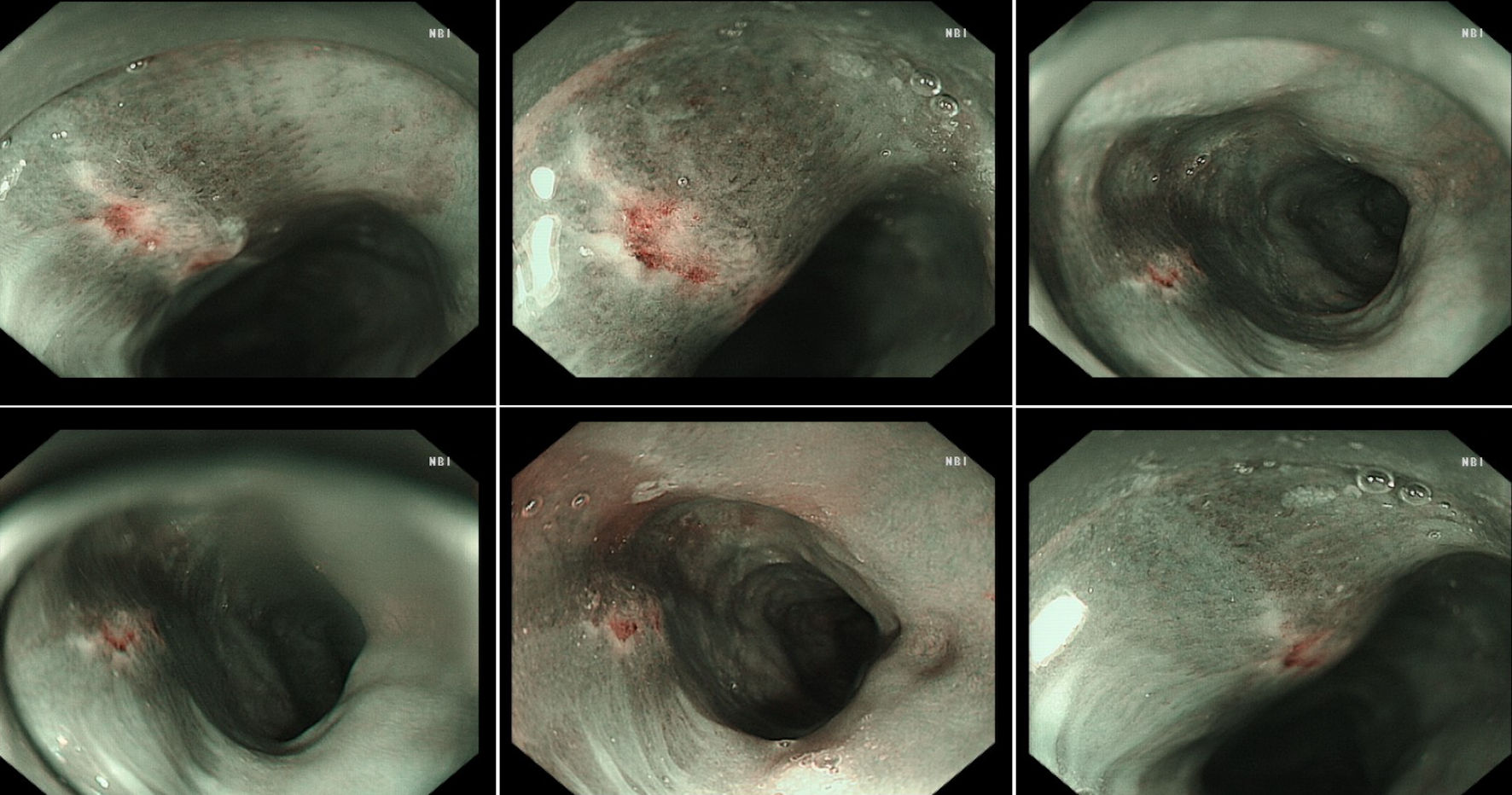 Click for large image | Figure 2. The boundary of the esophageal ulcer was visualized using magnifying endoscopy with narrow-band imaging (ME-NBI). ME-NBI showed the boundary of the esophageal ulcer with color transformation to hazel, and no abnormal blood vessel was observed, indicating that neither intestinal metaplasia nor cancerization occurred in the esophageal ulcer. |
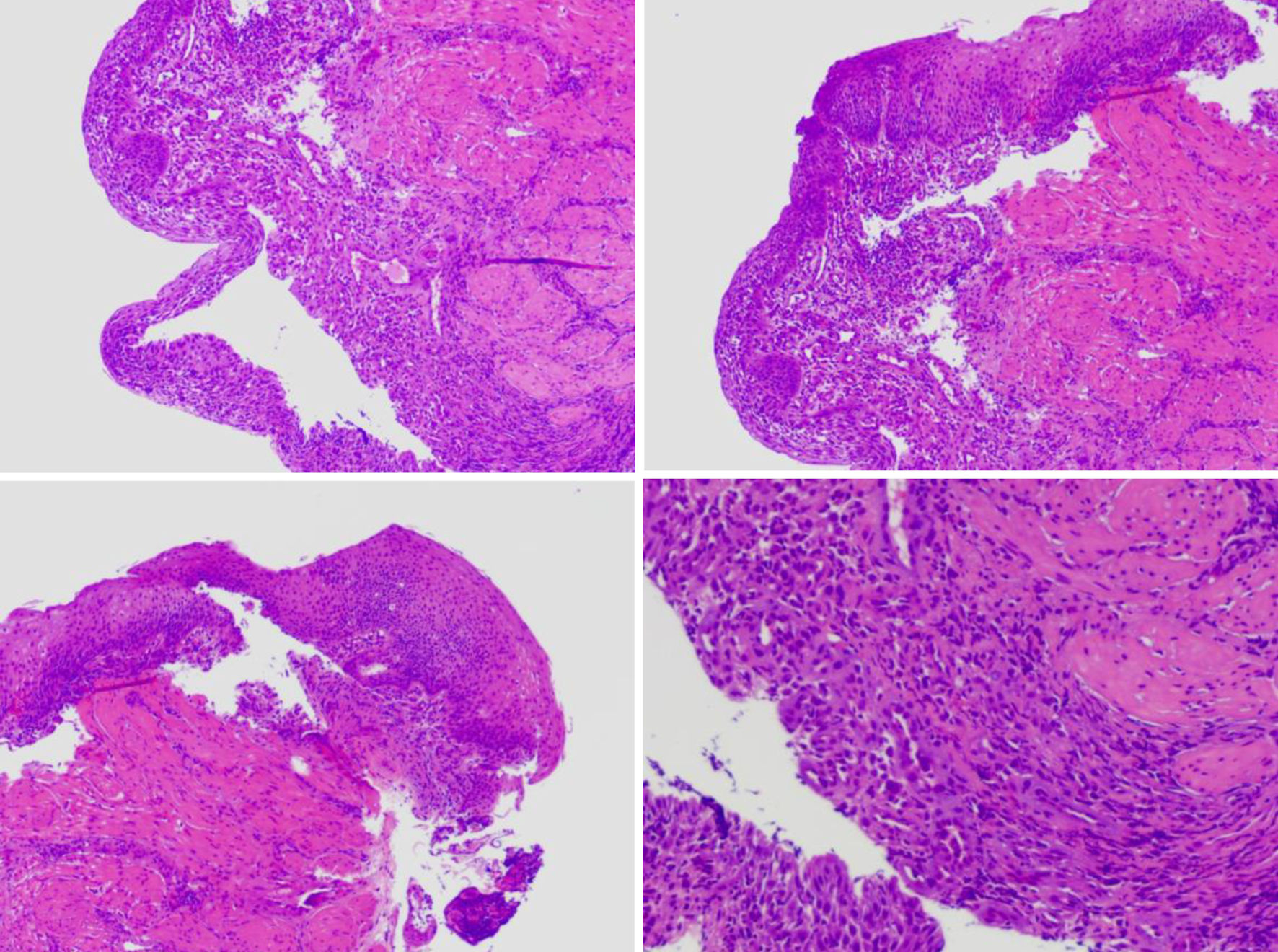 Click for large image | Figure 3. The esophageal biopsy revealed the pathological characteristic of the lesion using the optical microscope. |
The patient had no history of gastrointestinal disease or esophagus disorders, without intake of specific food prior to dysphagia. The esophageal ulcer was suspected by clinicians to be caused by ranibizumab. As a result, ranibizumab was discontinued. The patient received proton pump inhibitor (PPI) therapy to inhibit gastric acid secretion using ilaprazole sodium (10 mg, intravenous drip (ivgtt), once a day (qd), for 5 days, Livzon Pharmaceutical Group Inc., Zhuhai, China) and esomeprazole magnesium enteric-coated capsules (20 mg, oral (po), twice a day (bid), for 25 days, Chia Tai TianQing Pharmaceutical Group Co., Ltd. Lianyungang, China) on January 18, 2022. Meanwhile, Kangfuxin liquid (10 mL, po, three times a day (tid), for 30 days, Sichuan Gooddoctor Panxi Pharmaceutical Group, Xichang, China), as a traditional Chinese medicine (TCM), was used to promote healing of the esophageal ulcer. Five days after treatment, the dysphagia and retrosternal pain were dramatically alleviated. The patient was discharged from the hospital in the opinion of clinicians, with the remaining oral medications, and no longer need to receive ranibizumab intravitreal injection. In terms of follow-up, the patient continued to take the rest of oral medications according to medical advice, the dysphagia and retrosternal pain were gradually relieved in the following days. The clinical symptoms disappeared completely when the oral medications were used up.
The patient was admitted to the People’s Hospital of Ningdu County on March 10, 2022. Gastroscope showed the esophageal mucosa was smooth and normal in color, without covered white fibrinous tissue, indicating that the esophageal ulcer was cured. In terms of follow-up, the esophageal ulcer has not recurred so far (Fig. 4). Meanwhile, the patient has not received ranibizumab injection.
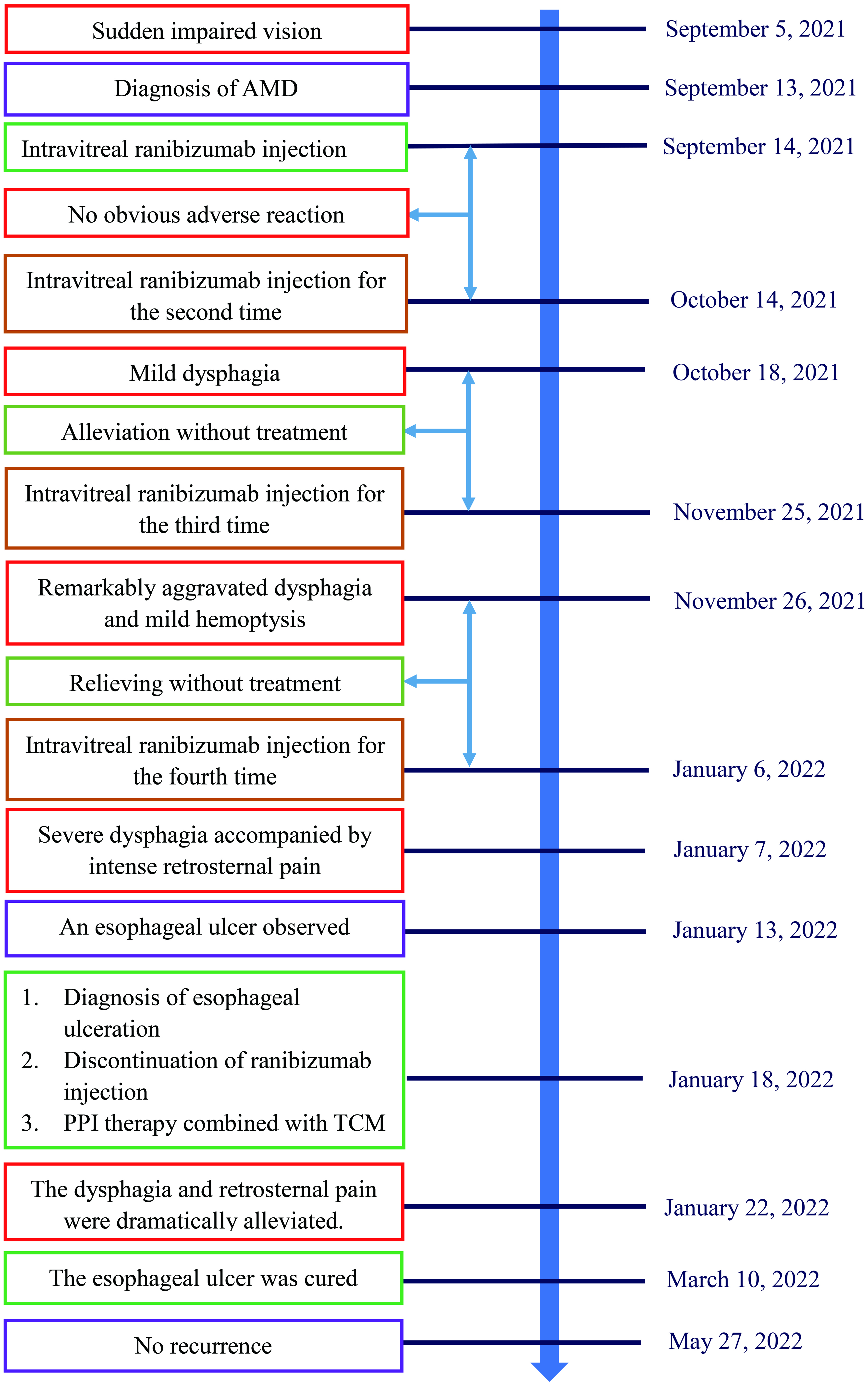 Click for large image | Figure 4. Timeline of ranibizumab-induced esophageal ulcer in a patient with age-related macular degeneration. AMD: age-related macular degeneration; PPI: proton pump inhibitor; TCM: traditional Chinese medicine. |
| Discussion | ▴Top |
Both ranibizumab and bevacizumab are monoclonal antibodies targeted against VEGF-A. However, bevacizumab was initially approved for the therapy of metastatic colorectal cancer while ranibizumab has long been used for the treatment of AMD [8, 9]. Interestingly, previous clinical studies demonstrated that bevacizumab and ranibizumab had similar efficacy as well as safety in the treatment of AMD, whereas ranibizumab has not been used to treat cancers so far [10, 11].
It has been reported that bevacizumab induced an esophageal ulcer in a 60-year-old woman. The women had difficulty in swallowing solid food after three cycles of regimens consisting of bevacizumab, irinotecan, 5-fluouracil and leucovorin. The esophageal ulcer was alleviated after sole discontinuation of bevacizumab [12]. In addition, two case reports revealed that two cancer patients experienced severe gastrointestinal ulceration after chemotherapy consisting of FOLFOX and bevacizumab, and the ulceration was considered to be associated with bevacizumab [13, 14]. In fact, Tol et al [15] reported that symptomatic gastrointestinal ulcers occurred in 1.3% of patients with advanced colorectal cancer (ACC) who received bevacizumab in combination of chemotherapy. Bergamo et al [16] found that 4.2% of patients experienced symptomatic anal ulceration after receiving bevacizumab. Moreover, vascularization of anal canal was notably inhibited in patients receiving bevacizumab, and timely discontinuation of bevacizumab protected patients from symptomatic anal ulcers. However, no report has shown that esophageal ulceration occurred in patients treated with ranibizumab. Noteworthily, bevacizumab was administrated through intravenous injection in the therapy of metastatic cancer, while ranibizumab was administrated by intravitreal injection in the treatment of ADM [3, 17]. Importantly, whether the chemotherapy had stimulative effects on bevacizumab-induced ulceration was unclear. In this case report, an esophageal ulcer was induced by ranibizumab alone.
VEGF is involved in angiogenesis that plays a crucial role in the repair of gastric ulcers [18]. It was reported that sofalcon exerted significant effects on gastric ulcer repair possibly by stimulating VEGF release in gastric fibroblasts [19]. Takahashi et al [20] demonstrated that VEGF mRNA was specifically expressed at gastric ulcer margins compared to normal gastric mucosae. Afterwards, Kim et al [21] found a novel single nucleotide polymorphism (SNP) in the VEGF gene that predicted the predisposition to gastroduodenal ulcers. In addition, Dai et al [22] speculated that Jianweiyuyang (JWYY) granules as TCM accelerated gastric ulcer healing potentially by stimulating angiogenesis and enhancing VEGF mRNA expression. These findings indicated that VEGF played a potential role in the development and progression of gastroduodenal ulcers.
Gastroesophageal reflux disease is the most common etiology of esophageal ulcers [23]. Moreover, the use of non-steroidal anti-inflammatory drugs or antibiotics (e.g., tetracycline and doxycycline) also can induce esophageal ulcers [7, 23]. The other causes of esophageal ulceration include the Candida, caustic injury, hiatal hernia, esophagitis, marginal ulcer, foreign body, human immunodeficiency virus (HIV), and herpes simplex virus (HSV) [6, 24].
With respect to the relationship between the esophageal ulcer and ranibizumab injection, dysphagia emerged three days after intravitreal ranibizumab injection, and aggravated after injecting ranibizumab again. Subsequently, dysphagia accompanied by hemoptysis or retrosternal pain occurred after duplicated administration of ranibizumab. The patient had no history of gastrointestinal disease or esophagus disorders prior to dysphagia. The esophageal ulcer was alleviated after ranibizumab discontinuation. Finally, the esophageal ulcer has not relapsed since permanent discontinuation of ranibizumab. Taken together, the esophageal ulcer was related to ranibizumab administration.
Conclusions
To our best knowledge, ranibizumab was firstly reported to induce an esophageal ulcer. This case report revealed a potential role of VEGF-A in the pathogenesis of esophageal ulceration. In view of the adverse reactions of bevacizumab in the digestive tract, it could be inferenced that VEGF-A might serve as a biomarker in the development and progression of ulceration in the digestive tract.
Acknowledgments
The authors thank all the medical staff for providing us help in the collection of clinical information of the patient.
Financial Disclosure
This work was supported by Ganzhou Municipal Science and Technology Bureau, Science and Technology Program of Ganzhou (No. 2022—YB1422).
Conflict of Interest
Ke Wei Zhu is employed by the Office of Pharmacovigilance of Guangzhou Baiyunshan Pharmaceutical Holding Co., Ltd. Baiyunshan Pharmaceutical General Factory, Guangzhou, China. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Informed Consent
The case report was approved by the independent Ethics Committee of Ganzhou People’s Hospital, Ganzhou, Jiangxi Province, China. Signed inform consent was obtained from the patient (No. 2022GZHRMYY-ICF-CR-05; signing data: January 22, 2022).
Author Contributions
All authors contributed to the study conception and design. LXQ, LJ, WJ, and GXF were responsible for collection of the case history and follow-up. ZKW wrote the manuscript, and LXQ was responsible for the submission. All authors reviewed the manuscript and agreed the final version of the case report to be published. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
| References | ▴Top |
- Ferro Desideri L, Barra F, Ferrero S, Traverso CE, Nicolo M. Clinical efficacy and safety of ranibizumab in the treatment of wet age-related macular degeneration. Expert Opin Biol Ther. 2019;19(8):735-751.
doi - Bressler NM. Age-related macular degeneration is the leading cause of blindness. JAMA. 2004;291(15):1900-1901.
doi - Dhoot DS, Kaiser PK. Ranibizumab for age-related macular degeneration. Expert Opin Biol Ther. 2012;12(3):371-381.
doi - Frampton JE. Ranibizumab: a review of its use in the treatment of neovascular age-related macular degeneration. Drugs Aging. 2013;30(5):331-358.
doi - Schmucker C, Ehlken C, Agostini HT, Antes G, Ruecker G, Lelgemann M, Loke YK. A safety review and meta-analyses of bevacizumab and ranibizumab: off-label versus goldstandard. PLoS One. 2012;7(8):e42701.
doi pubmed pmc - Higuchi D, Sugawa C, Shah SH, Tokioka S, Lucas CE. Etiology, treatment, and outcome of esophageal ulcers: a 10-year experience in an urban emergency hospital. J Gastrointest Surg. 2003;7(7):836-842.
doi - Dag MS, Ozturk ZA, Akin I, Tutar E, Cikman O, Gulsen MT. Drug-induced esophageal ulcers: case series and the review of the literature. Turk J Gastroenterol. 2014;25(2):180-184.
doi - Garcia J, Hurwitz HI, Sandler AB, Miles D, Coleman RL, Deurloo R, Chinot OL. Bevacizumab (Avastin(R)) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat Rev. 2020;86:102017.
doi - Ebneter A, Westenskow PD. Age-related macular degeneration and diabetic retinopathy. J Pers Med. 2022;12(4):581.
doi pubmed pmc - Comparison of Age-related Macular Degeneration Treatments Trials Research Group, Writing C, Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2020;127(4S):S135-S145.
doi - Solomon SD, Lindsley KB, Krzystolik MG, Vedula SS, Hawkins BS. Intravitreal bevacizumab versus ranibizumab for treatment of neovascular age-related macular degeneration: findings from a Cochrane systematic review. Ophthalmology. 2016;123(1):70-77.e71.
doi pubmed pmc - Meza-Junco J, Wong C, Fields A, Sawyer MB. Esophageal ulcer in a patient who received bevacizumab. Invest New Drugs. 2010;28(1):98-101.
doi - Rathur A, Al-Mohamad H, Steinhoff J, Walsh R. Chest pain from pneumopericardium with gastropericardial fistula. Case Rep Cardiol. 2021;2021:5143608.
doi pubmed pmc - Libanio D, Brandao C, Pimentel-Nunes P, Dinis-Ribeiro M. Perforated gastric ulcer associated with anti-angiogenic therapy. GE Port J Gastroenterol. 2017;24(6):285-287.
doi pubmed pmc - Tol J, Cats A, Mol L, Koopman M, Bos MM, van der Hoeven JJ, Antonini NF, et al. Gastrointestinal ulceration as a possible side effect of bevacizumab which may herald perforation. Invest New Drugs. 2008;26(4):393-397.
doi pubmed pmc - Bergamo F, Lonardi S, Salmaso B, Lacognata C, Battaglin F, Cavallin F, Saadeh L, et al. Angiogenesis inhibitors and symptomatic anal ulcers in metastatic colorectal cancer patients (*). Acta Oncol. 2018;57(3):412-419.
doi - Nie K, Zhang Z, You Y, Zhuang X, Zhang C, Ji Y. A randomized clinical study to compare intrapleural infusion with intravenous infusion of bevacizumab in the management of malignant pleural effusion in patients with non-small-cell lung cancer. Thorac Cancer. 2020;11(1):8-14.
doi pubmed pmc - Tarnawski AS. Cellular and molecular mechanisms of gastrointestinal ulcer healing. Dig Dis Sci. 2005;50(Suppl 1):S24-33.
doi - Takahashi M, Maeda S, Ogura K, Terano A, Omata M. The possible role of vascular endothelial growth factor (VEGF) in gastric ulcer healing: effect of sofalcone on VEGF release in vitro. J Clin Gastroenterol. 1998;27(Suppl 1):S178-S182.
doi - Takahashi M, Kawabe T, Ogura K, Maeda S, Mikami Y, Kaneko N, Terano A, et al. Expression of vascular endothelial growth factor at the human gastric ulcer margin and in cultured gastric fibroblasts: a new angiogenic factor for gastric ulcer healing. Biochem Biophys Res Commun. 1997;234(2):493-498.
doi - Kim YS, Park SW, Kim MH, Jang EJ, Park JS, Park SJ, Baik HW, et al. Novel single nucleotide polymorphism of the VEGF gene as a risk predictor for gastroduodenal ulcers. J Gastroenterol Hepatol. 2008;23(Suppl 2):S131-139.
doi - Dai XP, Li JB, Liu ZQ, Ding X, Huang CH, Zhou B. Effect of Jianweiyuyang granule on gastric ulcer recurrence and expression of VEGF mRNA in the healing process of gastric ulcer in rats. World J Gastroenterol. 2005;11(35):5480-5484.
doi pubmed pmc - Cohen DL, Bermont A, Richter V, Shirin H. Real world management of esophageal ulcers: analysis of their presentation, etiology, and outcomes. Acta Gastroenterol Belg. 2021;84(3):417-422.
doi - Wolfsen HC, Wang KK. Etiology and course of acute bleeding esophageal ulcers. J Clin Gastroenterol. 1992;14(4):342-346.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


