| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Original Article
Volume 16, Number 2, April 2023, pages 96-104
Is There a Difference in Adenoma Detection Rates According to Indication? An Experience in a Panamanian Colorectal Cancer Screening Program
Julio Zuniga Cisnerosa, c , Carlos Tunonb, Enrique Adamesa, b, Carolina Garciaa, Rene Riveraa, Eyleen Gonzaleza, Jan Cubillab, Luis Lambranob
aSchool of Medicine, University of Panama, Panama City, Panama
bDepartment of Gastroenterology, Santo Tomas Hospital, Panama City, Panama
cCorresponding Author: Julio Zuniga Cisneros, School of Medicine, University of Panama, Panama City, Panama
Manuscript submitted January 15, 2023, accepted March 23, 2023, published online April 28, 2023
Short title: Adenoma Detection Affected by Indication
doi: https://doi.org/10.14740/gr1599
| Abstract | ▴Top |
Background: The benefit of colorectal cancer screening in reducing cancer risk and related death is unclear. There are quality measure indicators and multiple factors that affect the performance of a successful colonoscopy. The main objective of our study was to identify if there is a difference in polyp detection rate (PDR) and adenoma detection rate (ADR) according to colonoscopy indication and which factors might be associated.
Methods: We conducted a retrospective review of all colonoscopies performed between January 2018 and January 2019, in a tertiary endoscopic center. All patients ≥ 50 years old scheduled for a nonurgent colonoscopy and screening colonoscopy were included. We stratified the total number of colonoscopies into two categories according to the indication: screening vs. non-screening, and then calculated PDR, ADR and serrated polyp detection rate (SDR). We also performed logistic regression model to identify factors associated with detecting polyps and adenomatous polyps.
Results: A total of 1,129 and 365 colonoscopies were performed in the non-screening and screening group, respectively. In comparison with the screening group, PDR and ADR were lower for the non-screening group (33% vs. 25%; P = 0.005 and 17% vs. 13%; P = 0.005). SDR was non-significantly lower in the non-screening group when compared with the screening group (11% vs. 9%; P = 0.53 and 22% vs. 13%; P = 0.007).
Conclusion: In conclusion, this observational study reported differences in PDR and ADR depending on screening and non-screening indication. These differences could be related to factors related to the endoscopist, time slot allotted for colonoscopy, population background, and external factors.
Keywords: Colonoscopy; Adenoma detection rate; Polyp detection rate; Serrated polyp detection rate
| Introduction | ▴Top |
Colorectal cancer (CRC) is the third leading cause of cancer death worldwide [1]. In the Republic of Panama, it is the fourth cause of mortality with most patients being diagnosed late with advanced disease [2]. Despite the known success and international recommendations that have led to early detection of CRC, screening colonoscopy is not routinely done in Panama [2]. A significant amount of colonoscopies are done after a symptom that warrants endoscopic evaluation appears. This often could result in procedures directed to the underlying symptom without enough emphasis or time dedicated to screen for polyps (i.e., potential CRC). For multiple reasons including costs and availability in our country, patients do not get screening colonoscopies following these diagnostic colonoscopies. Therefore, we wanted to evaluate whether there is a difference in adenoma detection rates (ADRs) between screening colonoscopies and those performed for other indications (lower gastrointestinal (GI) bleed, constipation, etc.).
ADR is considered to be the most important quality measure of a screening colonoscopy since it has been demonstrated as the only quality indicator that predicts independently interval CRC, with a decrease of 3% for each 1% increase in ADR [3, 4]. In addition, an inverse association between CRC mortality and ADR has been reported [3, 5, 6]. By comparison, polyp detection rate (PDR) has received attention as an alternative measure for ADR due to more convenient administrative process without dependence in pathology reports. Moreover, one study showed a strong correlation of 0.86 (P < 0.001) between ADR and PDR, findings supported by other studies [7-11]. Currently, PDR has no guidelines recommendation as a quality measure due to lack of strong evidence and prospective studies.
PDR and ADR have been reported to vary among endoscopist [12-14]. Some authors have even suggested a variation according to colonoscopy indication, with higher rates in surveillance and screening colonoscopy compared with non-screening colonoscopies [7, 10, 12]. In contrast, some researchers suggested that other ADR targets may be more appropriate, depending on local facilities, accessibility, and population background [15, 16]. However, recent guidelines have defined an overall aim of ≥ 30% ADR for colonoscopies in general, independent of the indication [17].
Recent studies have identified several factors that could affect ADR and might explain variations among different reports, such as local technological innovation, population background, the time slot for colonoscopy, local administrative factors, the experience of the endoscopist, among others [17-20].
The goal of this study was to identify differences in ADR and PDR between screening colonoscopy and non-screening colonoscopy, as well as to detect potential factors that might be associated with detection of polyps and adenoma.
| Materials and Methods | ▴Top |
Setting, subjects and materials
We conducted a retrospective review of all colonoscopies performed between January 2018 and January 2019, in a tertiary endoscopic center in Panama City. All patients ≥ 50 years old scheduled for a nonurgent colonoscopy and screening colonoscopy were included. The research protocol was approved by Hospital del Nino Bioethics Committee and conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Colonoscopies were performed at a single academic medical center by seven experienced gastroenterologists. All examinations were performed using high-definition colonoscopies CFQ 180 AL, CV-190 (Olympus America, Center Valley, PA).
Patient demographic information (age, sex, and first relative family history of CRC), colonoscopy indication, polyps identified including location, size, and the pathology report were collected. We classified polyp location as proximal or distal depending on the polyp’s relationship to the splenic flexure. All polyps resected were reviewed by a pathologist.
In regard to colonoscopy quality assessment measurements, cecal intubation rate (CIR), withdrawal time (WT), and bowel preparation using Boston Bowel Preparation Scale (BBPS) were recorded [21]. We stratified the total number of colonoscopies into two categories: screening vs. non-screening, and then calculated PDR, ADR, and serrated polyp detection rate (SDR) for each group and by sex according to guidelines recommendation, due to variation in the recommended detection rates for each sex [22].
All the polyps resected were processed in the pathology laboratory and employed strict quality control such as: 1) Verification of the correct sample processing following established protocol to guarantee a proper pathology interpretation; 2) Application of updated histopathological criteria to classify the polyps; and 3) A review by a second pathologist if any uncertainty of the pathologic diagnosis existed.
PDR was defined as the proportion of colonoscopies where ≥ 1 polyps were identified/total number of colonoscopies and expressed as percentages; ADR was defined as a proportion of colonoscopies in which ≥ 1 histologically confirmed adenomas were detected/total number of colonoscopies and expressed as percentages; SDR was defined as the proportion of colonoscopies where ≥ 1 histologically confirmed proximal serrated polyp was detected/total number of colonoscopies and expressed as percentages. Serrated polyp includes hyperplastic polyps (HPs), sessile serrated adenoma/polyps (SSA/Ps), and traditional serrated adenomas (TSAs) [23]. Non-classified polyps were defined as those that do not meet the criteria to be classified as serrated polyp or adenoma polyp. As a method to verify the histopathology report, these samples were reviewed by two different pathologists.
We only included patients in the screening group without prior colonoscopy or with no prior history of any previous polyps identified on previous screening colonoscopy. Non-screening indications included diverticular disease (DD), inflammatory bowel disease (IBD), chronic diarrhea (CD), constipation, anemia/weight loss (WL), low gastrointestinal bleeding (LGB), suspected colonic disease, and abdominal pain.
In our institution, the protocol for bowel preparation consists of a clear liquid diet for 12 h prior to colonoscopy and use of 4 L split-dose bowel preparation of polyethylene glycol, where one dose was taken 4 h prior to the study [24]. Given that the status of bowel preparation might affect PDR, ADR and SDR in a similar way, we did not exclude patients with poor bowel preparation (defined as BBPS < 6).
Statistical and data analysis
For the purpose of analysis, the data were divided into two comparative groups (screening colonoscopy vs. non-screening colonoscopy). Age, WT, and colonoscopy working time were classified as continuous data and presented as mean ± standard deviation (SD). Sex, CIR, BBPS, family history of colon cancer, dysplasia, colon cancer, angiodysplasia, diverticulosis, and hemorrhoids were classified as categorical data and summarized as frequencies and percentages (%).
We performed a Kolmogorov-Smirnov test to check for normal distribution of continuous data. Our analysis concluded our data were not normally distributed.
The differences between continuous variables were analyzed using Student’s t-tests for independent variables. Chi-squared test was used to analyze differences in distribution among categorical variables. Results were considered statistically significant for a P value < 0.05 (two-tailed).
We performed three models applying logistic multivariate regression analysis to identify factors associated to the detection of polyps, adenomatous polyps, and serrated polyps, each one individually and adjusted according to colonoscopies with a BBPS ≥ 6.
All the models included independent predictive variables: sex (male/female), age (years), first relative CRC family history (yes/no), WT (min), and cecal intubation (yes/no). The significance level of each model was established at 0.05.
All analyses were performed using Stata version 14.0 (StataCorp LLC, Lakeway Drive, Tx). Figures were designed using GraphPad Prism 9 (GraphPad Software Inc., San Diego, CA). The rate of incomplete data was overall small (< 5%).
| Results | ▴Top |
Patients, colonoscopy quality and histopathological findings
During the study period, 1,129 colonoscopies were performed in the non-screening group and 365 in the screening group. Screening group patients were slightly younger than non-screening patients (56.9 ± 0.42 vs. 60.7 ± 0.42; P = 0.003). Female sex was predominant in both groups with 74.5% (272/365) in the screening group and 66.7% (753/1,129) in the non-screening group. This sex difference was statistically significant (P = 0.004). Patient demographics, colonoscopy quality measures, and polyp histological features are described in Table 1. With respect to non-screening colonoscopy indications, abdominal pain, and LGB were the most common indications (Fig. 1).
 Click to view | Table 1. Characteristics of Patients, Colonoscopy Quality Measures and Polyps Resected According to Screening vs. Non-Screening Colonoscopy Indication |
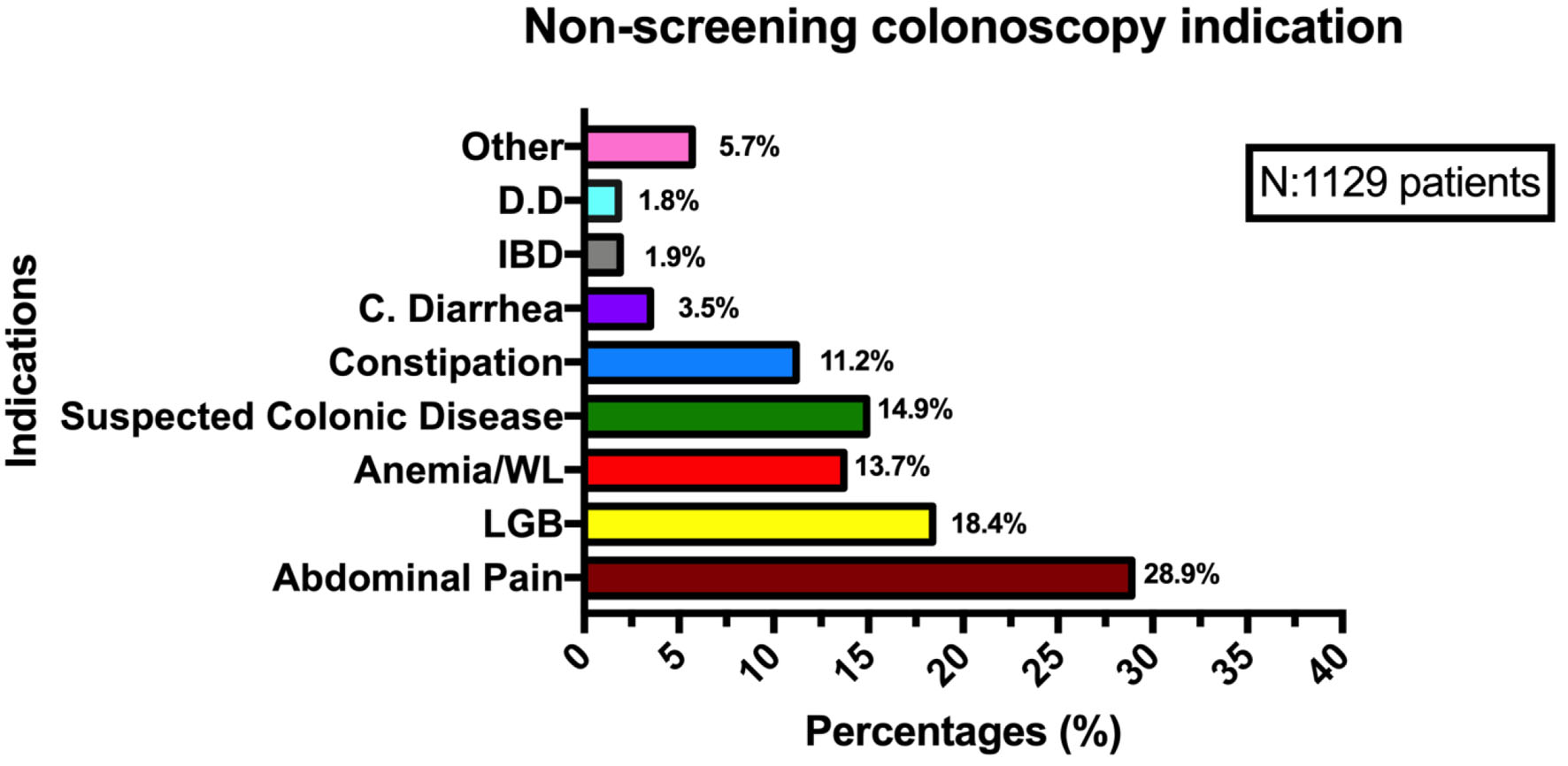 Click for large image | Figure 1. Non-screening colonoscopy indications. DD: diverticular disease; IBD: inflammatory bowel disease; C. diarrhea: chronic diarrhea; WL: weight loss; LGB: low gastrointestinal bleeding. |
Table 2 summarizes the histopathological characteristics of polyps resected in all colonoscopies performed. Adenomatous and hyperplasic polyps were the most frequently reported polyps according to the pathology. There were small differences in polyp location, totaling 40.6% (145/total) for distal location and 36.1% (129/total) for proximal location. In regard to serrated and traditional serrated polyps, the lack of pathology reports of these types of polyps was quite notable.
 Click to view | Table 2. Histological Features in Resected Polyp According to Size, Location, and Presence of Dysplasia |
CIR, WT, and colon preparation in screening vs. non-screening indication
The CIR proportion was similar in screening group (93.1%) vs. non-screening group (92.9%) with a no statistically significant difference (P = 0.174). In the WT analysis, there was a trend toward a shorter WT in those with non-screening colonoscopies (9.20 ± 0.24) vs. screening colonoscopies (10.19 ± 0.49) with a statistically significant difference (P = 0.046). We did not find any differences regarding the grade of preparation between screening and non-screening colonoscopy using the BBPS ≥ 6 (74.5% vs. 79.0%; P = 0.075) (Table 3).
 Click to view | Table 3. Colonoscopy Quality Indicators According to Indication of Screening vs. Non-Screening Colonoscopy |
PDR, ADR, and SDR in screening vs. non-screening indication
In comparison with the screening group, PDR and ADR were lower for the non-screening group (33% vs. 25%; P = 0.005 and 17% vs. 13%; P = 0.005). SDR was non-significantly lower in the non-screening group when compared to the screening group (11% vs. 9%; P = 0.53 and 22% vs. 13%; P = 0.007).
In comparison with the screening group, PDR, ADR, and SDR in female patients were lower for the non-screening group (28% vs. 23%; P = 0.036, 15% vs. 12%; P = 0.040, and 10% vs. 9%; P = 0.077, respectively). In addition, PDR, ADR, and SDR in male patients were significantly lower in non-screening group when compared with screening group (43% vs. 25%; P = 0.005, 22% vs. 13%; P = 0.007, and 13% vs. 8%; P = 0.032) (Fig. 2).
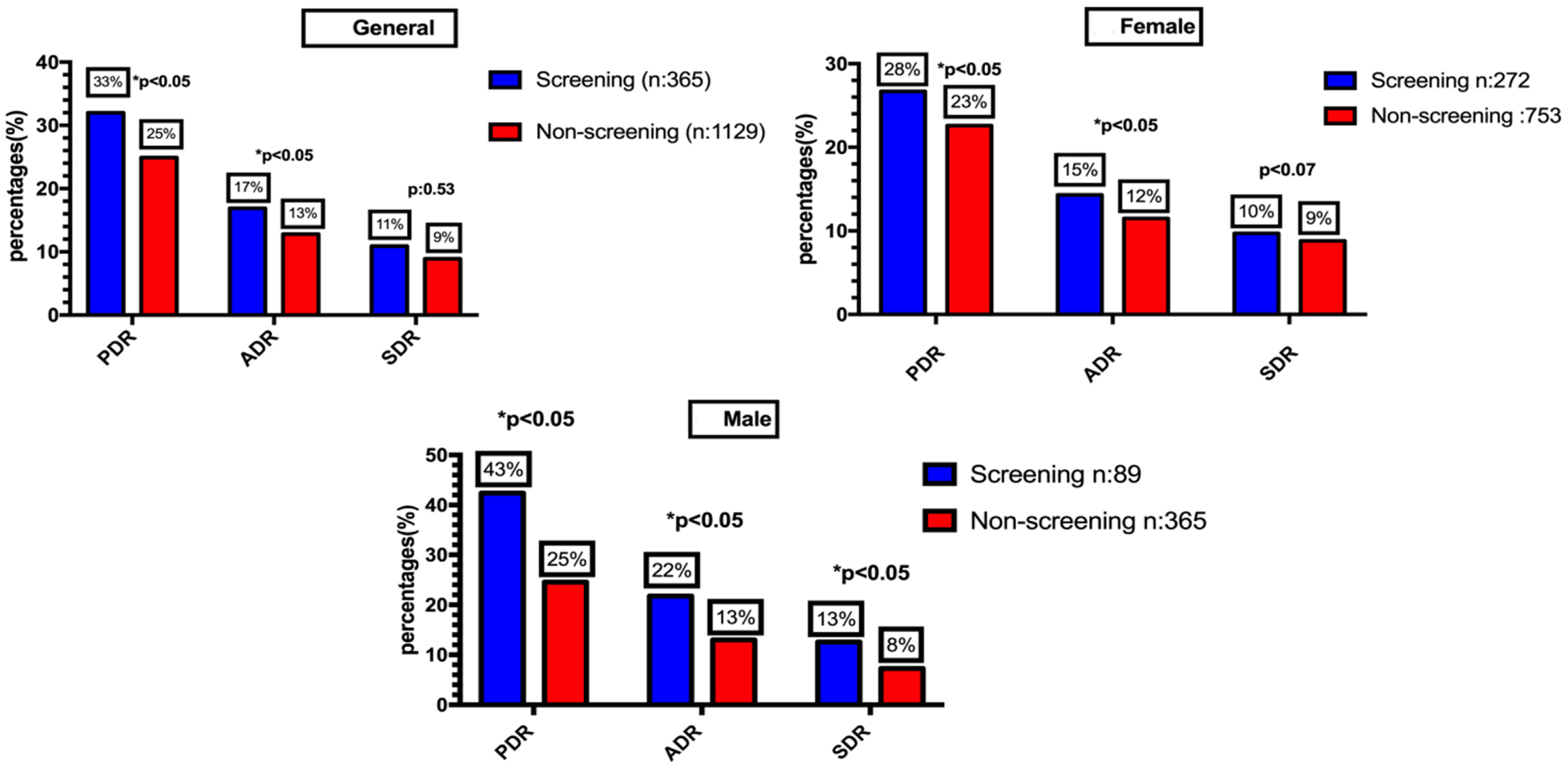 Click for large image | Figure 2. Polyp detection rate, adenoma detection rate and serrated polyp detection rate according to sex and indication of screening vs. non-screening colonoscopy. |
Multivariable logistic regression model
Logistical regression analysis demonstrated in the polyp detection model that male sex (odds ratio (OR): 1.35 (confidence interval (CI): 1.07 - 1.84)), age (OR: 1.04 (CI: 1.02 - 1.05)), colonoscopy screening indication (OR: 1.44 (CI: 1.09 - 1.88)), and WT (OR: 1.19 (CI: 1.16 - 1.22)) were the only variables that significantly impacted PDR (Fig. 3a).
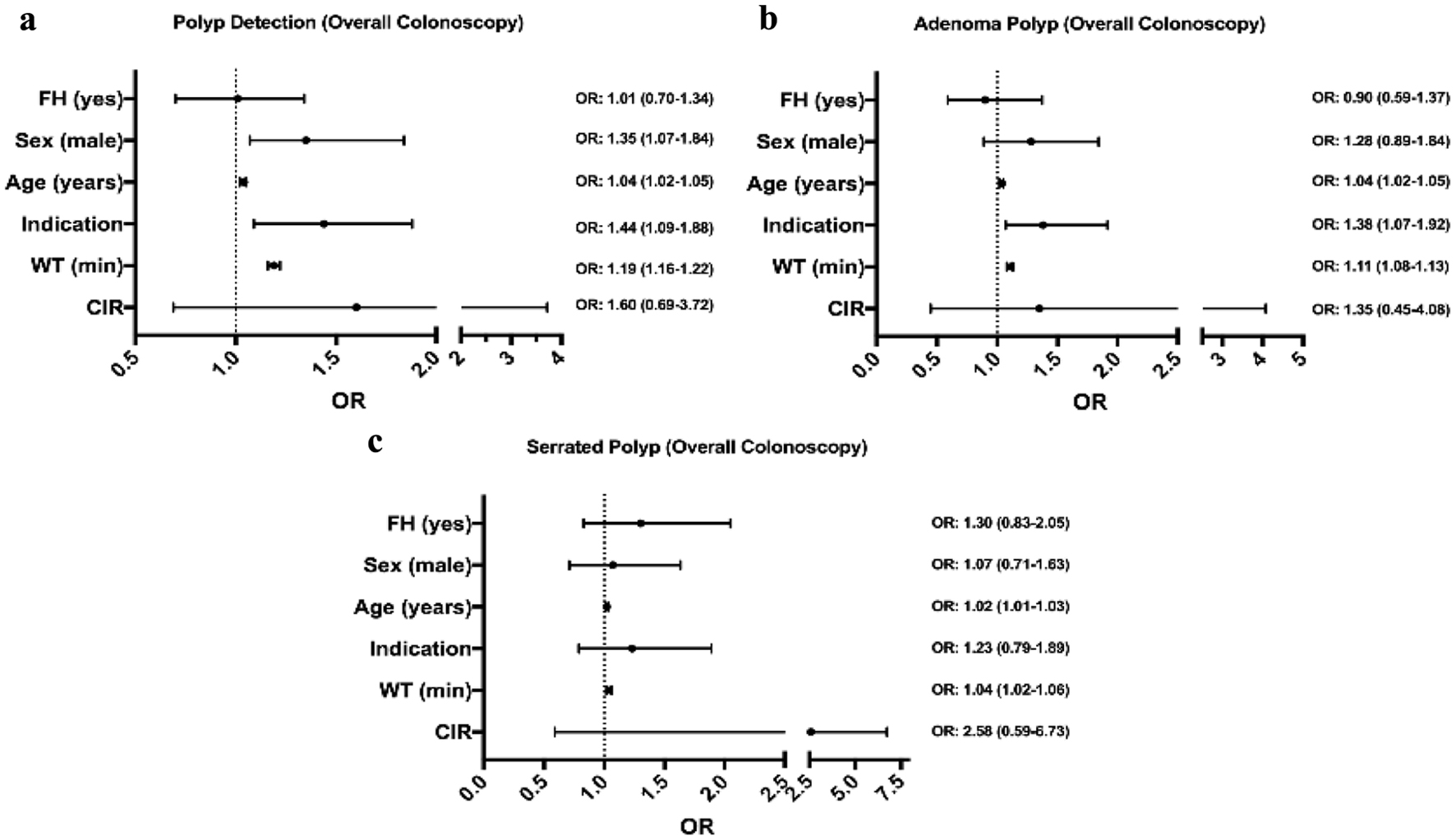 Click for large image | Figure 3. Multivariable logistic regression model predicting detection of polyps (a), adenomatous polyps (b), and serrated polyps (c) according to overall colonoscopies model and adjusted by Boston Bowel Preparation Scale. FH: family history of colon cancer; WT: withdrawal time in minutes; CIR: cecal intubation rate; OR: odds ratio values (mean and 95% confidence interval). |
In relation to the adenoma detection model, WT (OR: 1.11 (CI: 1.08 - 1.13)), indication (OR: 1.38 (CI: 1.07 - 1.92)), and age (OR: 1.04 (CI: 1.02 - 1.05)) were the only variables that significantly impacted ADR (Fig. 3b).
When we analyzed the SDR model, only WT (OR: 1.04 (CI: 1.02 - 1.06)) and age (OR: 1.01 (CI: 1.02 - 1.03)) were predictive of serrated polyp detection (Fig. 3c).
Family history of CRC was not associated with increased PDR, ADR, and SDR.
| Discussion | ▴Top |
The present study assessed the influence of colonoscopy indication and colonoscopy quality measurements in the detection of polyps in a tertiary endoscopic center. This analysis showed statistically significant differences in the PDR and ADR between screening and non-screening colonoscopy, regardless of sex. We also observed that population background such as age, sex (male), and factors related to colonoscopy such as WT and screening indication increased the odds to detect a polyp or adenoma.
We calculated the ADR according to sex as suggested by current guidelines. The screening group almost reached the standard recommended values in male (25%) and female patients (15%) with 22.4% and 14.8% accordingly; furthermore, a significant difference in the ADR was observed when compared to non-screening group in male (13.2%) and female patient (11.5%), a finding reported in previous studies [7, 10, 25]. Measuring the ADR is a priority for colonoscopy quality improvement in a CRC screening program, although we could not reach the recommended standard value for ADR, probably other factors could affect this quality measurements such as bowel preparation. In our study up to 25.5% in the screening group and 21.0% in the non-screening group have inadequate bowel preparation (BPPS < 6), in both groups ≤ 90% as recommended by recent guidelines [26]. Other possible factor that affects the ADR could be related to sex. In our study, female sex was more common without significant difference by age when compared with male (P = 0.093). Another factor might be the number of screening endoscopy performed in our study. This is in line with other reports highlighting the importance of a large sample size to assess a reliable ADR [22, 27].
The screening PDRs in our study for male and female were 43.4% and 27.6%, respectively. As suggested by other studies, PDR is a surrogate measurement that correlates with ADR [11, 28, 29]. William et al suggested that PDRs of 40% and 30% were correlated with ADR targets of 25% for male and 15% for female, accordingly [11, 28]. In our study, we observed an important difference in the detection rates when compared with non-screening colonoscopy, 25.5% in male and 22.6% in female. As PDR has been suggested as a substitute for the cumbersome ADR, we could not find a prospective study that evaluates PDR as a quality measure. There is a lack of good quality evidence, and more studies are needed.
In our study, WT, screening indication, and age were associated with an increased odds of PDR and ADR. Our data showed that despite our patient bowel preparation cleanliness, it did not reach standard goals (> 90%) overall in both group and there was no difference in CIR between screening and non-screening group, but we observed a significant difference in the ADR and PDR between screening and non-screening colonoscopy.
As it has been demonstrated in previous studies, there is a great variability in detecting serrated lesions among endoscopists [30-32]. One study reported a variation ranging from 1% to 18% among 15 academic gastroenterologists [31]. In our study, the detection rate of serrated polyps varied from 4.8% to 10.8% among seven endoscopists. In addition, we observed a statistically significant difference in male SDR between screening and non-screening group.
Endoscopic detections of serrated polyp are more challenging because of the serrated polyp’s endoscopic features and difficulties with histological interpretation. In our study, almost 40% of serrated polyps were detected in the proximal colon; however, all polyps were hyperplasic unlike other studies where an important proportion of proximal serrated polyps were SSA/Ps and TSAs [30, 33, 34]. We think this finding could be associated with less experience in the identification of serrated polyps by non-gastrointestinal pathologist and with endoscopist experience in the resection of serrated polyp as was reported in other reports [30, 34-37]. Studies focused on serrated polyp detection including endoscopy resection technique and histopathological interpretation are necessary to determine factor associated with SDR and the role as a quality metric in a CRC screening program.
In Panama, according to previous reports, there was a stable trend in the incidence rates and mortality of CRC for the period between 2001 and 2011 [38]. Despite the fact that there is not a public health policy for routine CRC screening, most endoscopic centers in tertiary and secondary level hospitals offer screening and surveillance. In guidelines and publications investigating quality measures for CRC screening program, evaluating a program and identifying factors related to colonoscopy good quality performance have been associated with an inverse relationship with post-colonoscopy cancer [22, 27, 39-41]. We encourage that each program should analyze its performance and identify strategies to improve quality metrics since every measurement, mainly ADR, could be affected by background feature of the population, factors related to the endoscopist, quality of colonoscopy, colonoscopy allotted time, equipment (technology), and institution related factor [17-19, 33, 42]. For example, in our study, increasing time of colonoscopy in non-screening colonoscopy, improving bowel preparation cleanliness and identifying with the pathology department as a whole team strategies to detect serrated polyp could have an important impact in our quality metrics.
Our study has strengths and limitations. The use of a community-based cohort without exclusion of patients based on comorbid illnesses or inadequate bowel preparation might reflect the prevalence of adenomas observed in general medical practice. We verified each pathology report for all polyps sent for histology interpretation providing complete pathology data for this group of patients. A limitation of our study includes the collection of data from one institution, which may limit the generalizability of study findings. In our institution, all pathology specimens were analyzed by general pathologists. We do not have pathologist specializing in gastrointestinal pathology. The analysis of PDR, ADR and SDR was generalized as an institution and not by endoscopist in all colonoscopies performed in an annual basis. The lower number obtained in screening colonoscopies vs. other indications might be related to the lack of formal national policies about CRC screening program in Panama.
Another limitation potentially influencing the results could be related to the impact of lifestyle behavior and socioeconomic differences in the ADRs between patients in the screening and non-screening groups. We did not quantify these variables to analyze their influence on the results. However, the population studied came from the same socioeconomic status which might reduce a possible bias.
To our knowledge, this the first report in a Central American population describing colonoscopy quality assessment differences according to clinical indication. In conclusion, this observational study reported differences in PDR and ADR depending on screening and non-screening indication. These differences could be associated to factors related to the endoscopist, time slot allotted for colonoscopy, population background and external factors including pathology interpretation. We suggest that each institution with a CRC screening program should propose studies focused on the identification of factors associated with increased ADR, and this strategy might be more associated with better results in long terms to achieve ADR targets as suggested by guidelines. We hope our study can be used by other institutions to make them evaluate their ADR and improve CRC detection rates.
Acknowledgments
We would like to thank Pathology Department for their support in reporting polyps resected.
Financial Disclosure
There was no financial support to report.
Conflict of Interest
There was no conflict of interest to report.
Informed Consent
Not applicable.
Author Contributions
JZ, CT, EA, CG, RR, EG, JC and LL conceptualized this project and participate in the study conduct, data collection, data analyses and writing. Each author read and approved the final manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol. 2019;14(2):89-103.
doi pubmed pmc - Politis M, Higuera G, Chang LR, Gomez B, Bares J, Motta J. Trend analysis of cancer mortality and incidence in Panama, using joinpoint regression analysis. Medicine (Baltimore). 2015;94(24):e970.
doi pubmed pmc - Corley DA, Jensen CD, Marks AR, Zhao WK, Lee JK, Doubeni CA, Zauber AG, et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370(14):1298-1306.
doi pubmed pmc - Kaminski MF, Regula J, Kraszewska E, Polkowski M, Wojciechowska U, Didkowska J, Zwierko M, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010;362(19):1795-1803.
doi - Wieszczy P, Regula J, Kaminski MF. Adenoma detection rate and risk of colorectal cancer. Best Pract Res Clin Gastroenterol. 2017;31(4):441-446.
doi - Kaminski MF, Wieszczy P, Rupinski M, Wojciechowska U, Didkowska J, Kraszewska E, Kobiela J, et al. Increased rate of adenoma detection associates with reduced risk of colorectal cancer and death. Gastroenterology. 2017;153(1):98-105.
doi - Boroff ES, Disbrow M, Crowell MD, Ramirez FC. Adenoma and Polyp Detection Rates in Colonoscopy according to Indication. Gastroenterol Res Pract. 2017;2017:7207595.
doi pubmed pmc - Murphy B, Myers E, O'Shea T, Feeley K, Waldron B. Correlation between adenoma detection rate and polyp detection rate at endoscopy in a non-screening population. Sci Rep. 2020;10(1):2295.
doi pubmed pmc - Zorron Cheng Tao Pu L, Singh G, Rana K, Nakamura M, Yamamura T, Krishnamurthi S, Ovenden A, et al. Polyp detection rate as a surrogate for adenoma and sessile serrated adenoma/polyp detection rates. Gastrointest Tumors. 2020;7(3):74-82.
doi pubmed pmc - Hoff G, Botteri E, Hoie O, Garborg K, Wiig H, Huppertz-Hauss G, Moritz V, et al. Polyp detection rates as quality indicator in clinical versus screening colonoscopy. Endosc Int Open. 2019;7(2):E195-E202.
doi pubmed pmc - Williams JE, Le TD, Faigel DO. Polypectomy rate as a quality measure for colonoscopy. Gastrointest Endosc. 2011;73(3):498-506.
doi - Imperiale TF, Glowinski EA, Juliar BE, Azzouz F, Ransohoff DF. Variation in polyp detection rates at screening colonoscopy. Gastrointest Endosc. 2009;69(7):1288-1295.
doi - Bretagne JF, Hamonic S, Piette C, Manfredi S, Leray E, Durand G, Riou F. Variations between endoscopists in rates of detection of colorectal neoplasia and their impact on a regional screening program based on colonoscopy after fecal occult blood testing. Gastrointest Endosc. 2010;71(2):335-341.
doi - Ricci E, Hassan C, Petruzziello L, Bazzoli F, Repici A, Di Giulio E. Inter-centre variability of the adenoma detection rate: a prospective, multicentre study. Dig Liver Dis. 2013;45(12):1022-1027.
doi - Barret M, Boustiere C, Canard JM, Arpurt JP, Bernardini D, Bulois P, Chaussade S, et al. Factors associated with adenoma detection rate and diagnosis of polyps and colorectal cancer during colonoscopy in France: results of a prospective, nationwide survey. PLoS One. 2013;8(7):e68947.
doi pubmed pmc - Wang H, Wang P, Liu X, Li L, Xiao X, Liu P, Zhang D, et al. Factors predicting the colorectal adenoma detection rate in colonoscopic screening of a Chinese population: A prospective study. Medicine (Baltimore). 2019;98(15):e15103.
doi pubmed pmc - Atkins L, Hunkeler EM, Jensen CD, Michie S, Lee JK, Doubeni CA, Zauber AG, et al. Factors influencing variation in physician adenoma detection rates: a theory-based approach for performance improvement. Gastrointest Endosc. 2016;83(3):617-626.e612.
doi pubmed pmc - Matyja M, Pasternak A, Szura M, Wysocki M, Pedziwiatr M, Rembiasz K. How to improve the adenoma detection rate in colorectal cancer screening? Clinical factors and technological advancements. Arch Med Sci. 2019;15(2):424-433.
doi pubmed pmc - Rex DK. Polyp detection at colonoscopy: Endoscopist and technical factors. Best Pract Res Clin Gastroenterol. 2017;31(4):425-433.
doi - Pullens HJ, Siersema PD. Quality indicators for colonoscopy: Current insights and caveats. World J Gastrointest Endosc. 2014;6(12):571-583.
doi pubmed pmc - Lai EJ, Calderwood AH, Doros G, Fix OK, Jacobson BC. The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc. 2009;69(3 Pt 2):620-625.
doi pubmed pmc - Kaminski MF, Thomas-Gibson S, Bugajski M, Bretthauer M, Rees CJ, Dekker E, Hoff G, et al. Performance measures for lower gastrointestinal endoscopy: a European Society of Gastrointestinal Endoscopy (ESGE) Quality Improvement Initiative. Endoscopy. 2017;49(4):378-397.
doi - Rosty C, Hewett DG, Brown IS, Leggett BA, Whitehall VL. Serrated polyps of the large intestine: current understanding of diagnosis, pathogenesis, and clinical management. J Gastroenterol. 2013;48(3):287-302.
doi pubmed pmc - Menees SB, Kim HM, Schoenfeld P. Split-dose bowel preparation improves adequacy of bowel preparation and gastroenterologists' adherence to National Colorectal Cancer Screening and Surveillance Guidelines. World J Gastroenterol. 2018;24(6):716-724.
doi pubmed pmc - Saini SD, Kim HM, Schoenfeld P. Incidence of advanced adenomas at surveillance colonoscopy in patients with a personal history of colon adenomas: a meta-analysis and systematic review. Gastrointest Endosc. 2006;64(4):614-626.
doi - Keswani RN, Crockett SD, Calderwood AH. AGA Clinical Practice Update on Strategies to Improve Quality of Screening and Surveillance Colonoscopy: Expert Review. Gastroenterology. 2021;161(2):701-711.
doi - Fayad NF, Kahi CJ. Colonoscopy quality assessment. Gastrointest Endosc Clin N Am. 2015;25(2):373-386.
doi - Williams JE, Holub JL, Faigel DO. Polypectomy rate is a valid quality measure for colonoscopy: results from a national endoscopy database. Gastrointest Endosc. 2012;75(3):576-582.
doi pubmed pmc - Fayad NF, Kahi CJ. Quality measures for colonoscopy: a critical evaluation. Clin Gastroenterol Hepatol. 2014;12(12):1973-1980.
doi - Hetzel JT, Huang CS, Coukos JA, Omstead K, Cerda SR, Yang S, O'Brien MJ, et al. Variation in the detection of serrated polyps in an average risk colorectal cancer screening cohort. Am J Gastroenterol. 2010;105(12):2656-2664.
doi - Kahi CJ, Hewett DG, Norton DL, Eckert GJ, Rex DK. Prevalence and variable detection of proximal colon serrated polyps during screening colonoscopy. Clin Gastroenterol Hepatol. 2011;9(1):42-46.
doi - de Wijkerslooth TR, Stoop EM, Bossuyt PM, Tytgat KM, Dees J, Mathus-Vliegen EM, Kuipers EJ, et al. Differences in proximal serrated polyp detection among endoscopists are associated with variability in withdrawal time. Gastrointest Endosc. 2013;77(4):617-623.
doi - Niv Y. Changing pathological diagnosis from hyperplastic polyp to sessile serrated adenoma: systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2017;29(12):1327-1331.
doi - Jaravaza DR, Rigby JM. Hyperplastic polyp or sessile serrated lesion? The contribution of serial sections to reclassification. Diagn Pathol. 2020;15(1):140.
doi pubmed pmc - Sanaka MR, Gohel T, Podugu A, Kiran RP, Thota PN, Lopez R, Church JM, et al. Adenoma and sessile serrated polyp detection rates: variation by patient sex and colonic segment but not specialty of the endoscopist. Dis Colon Rectum. 2014;57(9):1113-1119.
doi - Bordacahar B, Barret M, Terris B, Dhooge M, Dreanic J, Prat F, Coriat R, et al. Sessile serrated adenoma: from identification to resection. Dig Liver Dis. 2015;47(2):95-102.
doi - Gupta V, East JE. Optimal endoscopic treatment and surveillance of serrated polyps. Gut Liver. 2020;14(4):423-429.
doi pubmed pmc - Politis M. Trends in cancer mortality and incidence in Panama. n.d. http://www.gorgas.gob.pa/SIGCANCER/publicaciones/CANCER_TRENDS.pdf (accessed April 4, 2021).
- Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394(10207):1467-1480.
doi - Larsen MB, Njor S, Ingeholm P, Andersen B. Effectiveness of colorectal cancer screening in detecting earlier-stage disease-a nationwide cohort study in Denmark. Gastroenterology. 2018;155(1):99-106.
doi - European Colorectal Cancer Screening Guidelines Working Group, von Karsa L, Patnick J, Segnan N, Atkin W, Halloran S, Lansdorp-Vogelaar I, et al. European guidelines for quality assurance in colorectal cancer screening and diagnosis: overview and introduction to the full supplement publication. Endoscopy. 2013;45(1):51-59.
doi pubmed pmc - Kaminski MF, Thomas-Gibson S, Bugajski M, Bretthauer M, Rees CJ, Dekker E, Hoff G, et al. Performance measures for lower gastrointestinal endoscopy: a European Society of Gastrointestinal Endoscopy (ESGE) quality improvement initiative. United European Gastroenterol J. 2017;5(3):309-334.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


