| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Original Article
Volume 15, Number 3, June 2022, pages 120-126
Investigating Defects of Esophageal Motility in Lung Transplant Recipients
Jordan Burlena, Suma Chennubhotlab, Shifat Ahmedc, Sarah Landesd, Allan Ramireze, Abigail M. Stockere, Thomas L. Abelle, f
aDivision of Gastroenterology, Department of Internal Medicine, The Ohio State University, Columbus, OH, USA
bDivision of Gastroenterology, Department of Internal Medicine, St. Elizabeth Healthcare, Crestview Hills, KY, USA
cDivision of Gastroenterology, Department of Internal Medicine, Arizona Digestive Health, Phoenix, AZ, USA
dDivision of Gastroenterology, Department of Internal Medicine, Baptist Health, Louisville, KY, USA
eDivision of Gastroenterology, Hepatology and Nutrition, Department of Internal Medicine, University of Louisville, Louisville, KY, USA
fCorresponding Author: Thomas L. Abell, Division of Gastroenterology, Hepatology and Nutrition, University of Louisville, Louisville, KY 40202, USA
Manuscript submitted January 10, 2022, accepted March 30, 2022, published online June 22, 2022
Short title: GI Motility in Lung Transplant
doi: https://doi.org/10.14740/gr1501
| Abstract | ▴Top |
Background: Lung transplant patients are at risk of developing chronic lung allograft dysfunction (CLAD) of which bronchitis obliterans syndrome (BOS) is the most common. These patients also are noted to develop gastrointestinal (GI) disease. Gastroesophageal reflux disease (GERD) is implicated in BOS, and diagnosis and treatment of GERD may help to decrease incidence of BOS.
Methods: A total of 131 lung transplant recipients with post-transplant evaluation between 2012 and 2019 were studied. Of 60 post-transplant evaluations with at least 6 months of post-transplant follow-up that included impedance testing, high-resolution manometry (HRM), and pH testing, procedures were performed according to recognized standards.
Results: Of 60 patients, 56 (93%) were alive at 1-year post-transplant. The patients were found to have high rates of GI motility diseases: 37 patients (62%) had abnormal impedance testing, 50 patients (83%) had abnormal HRM results, 22 patients (37%) had abnormal pH test results. There was associated high rejection rates in patients with abnormal esophageal motility. There were 37 patients that had abnormal impedance test results and of those 25 patients (67%) developed rejection. Fifty patients had abnormal post-transplant HRM studies, 33 (66%) had an acute cellular rejection episode. Twenty-two patients had abnormal pH results, with 14 (63%) having an acute cellular rejection.
Conclusions: Patients undergoing lung transplantation were found to have increased incidence of abnormal GI motility studies of the esophagus. These patients were further found to have increased rejection rates and BOS which has been associated with worsened mortality. Developing a formalized pre- and post-transplant motility study process, using evolving technologies for these patients, may provide guidance of at-risk patients for CLAD and early treatment to prevent CLAD.
Keywords: Motility; Lung transplant; Reflux; Impedance; Manometry
| Introduction | ▴Top |
In 2017, a total of 34,769 organ transplants were performed in the USA, of which 2,449 were lung transplants, as noted by the United Network for Organ Sharing. Microaspiration and gastroesophageal reflux disease (GERD) have been implicated in the development of allograft rejection [1-3]. Several studies have shown the benefit of fundoplication in preventing reflux and slowing decline of transplant function [4, 5]. It is estimated that over 50% of lung transplant patients will develop allograft rejection, with the most well-known subtype being bronchiolitis obliterans syndrome (BOS), following transplant [6, 7]. This risk increases at 5 and 10 years, where the prevalence increases to 60% and 80%, respectively [8, 9]. Following histopathological confirmation of BOS, patients have a 5-year survival rate of only 30-40%, which is 20-40% lower than patients without BOS [10]. It is theorized that, GERD-induced innate immune system activation causes inflammation resulting in increased fibrosis of the bronchioles [10].
The onset of GERD is often before lung transplant surgery. It is common with patients with end-stage pulmonary disease secondary to cystic fibrosis and idiopathic pulmonary fibrosis [1]. In this population of patients, there has been a demonstrated survival advantage for those treated with early fundoplication [11, 12].
Lung transplant recipients are noted to develop GERD and a variety of dysmotility disorders. Post-transplant abdominal symptoms are often nonspecific and related to post-transplant immunosuppression, resulting in peptic ulcer disease, cytomegalovirus, and pancreatitis [13]. Gastroparesis is a condition that presents with nonspecific symptoms, including nausea, vomiting, epigastric fullness, anorexia, and dyspepsia [13]. Postoperative onset of GERD may be a result of accidental vagal nerve interruption causing gastroparesis [10].
Disorders of upper gut motility are thought to play a role in chronic lung allograft dysfunction (CLAD) (BOS in particular) amongst lung transplant patients. The goal of this study is to explore the characteristics of esophageal testing in patients with lung transplantation. Motility and pH-impedance testing is becoming important in lung transplantation candidate evaluations and follow-up assessments without a formalized approach to testing and management. By recognizing the risks posed by undiagnosed and possibly preventable dysmotility and reflux issues, decreased CLAD incidence may be achieved.
| Materials and Methods | ▴Top |
This study evaluated 131 patients, of which 60 patients had post-transplant evaluation between 2012 and 2019. All patients had at least 6 months of post-transplant follow-up. Among the 60 recipients (24 male, 36 female, 56 Caucasian, two Middle-Eastern, two African American; mean age at time of gastric studies was 58.5 ± 11.4 years), the indication for transplant included idiopathic pulmonary fibrosis, end-stage chronic obstructive pulmonary disease (COPD), or cystic fibrosis. Six patients received a single right lung transplant, 12 received a single left lung transplant, and 42 received a bilateral lung transplant. All patients received a uniform immunosuppression regimen: induction with basiliximab; tacrolimus to maintain a target trough level 10 - 12 ng/mL; prednisone was initiated at 20 mg tapered to 5 mg after the first year; and mycophenolate mofetil 1,000 mg, twice a day (BID) to maintain a white blood cell (WBC) > 3,000. Median time from transplant to gastrointestinal evaluation was 4.3 ± 4.96 months (range 0.69 - 25.21 months). Thirty-three patients had a prior clinical diagnosis of GERD, three with gastroparesis, and two with a history of esophageal dysmotility prior to lung transplantation.
All variables were binary in scope and summarized with counts and percentages. Logistic regression was used to derive adjusted odds ratios and 95% confidence intervals for the relationship between a rejection diagnosis and abnormal impedance, high-resolution manometry (HRM), and pH studies and RR calculations were done with Fisher’s exact test using R statistical software.
All patients received combined pH-impedance testing and HRM postoperatively. Combined impedance and pH testing was performed by placing an impedance probe transnasally and then into the esophagus. These sensors were 17, 15, 9, 7, 5 and 3 cm proximal to the lower esophageal sphincter (LES) to measure esophageal impedance before, during and after a high ion containing liquid bolus. Waveforms were stored and analyzed with dedicated software. Two pH sensors were placed 5 cm proximal and 10 cm distal to the LES. The studies were read and interpreted by trained gastroenterologists with expertise in gastrointestinal motility.
HRM was performed by passing a catheter transnasally into the esophagus. HRM used sensors placed every 1 cm in the esophagus. All anti-acid medications were held for 2 weeks prior to all gastrointestinal studies. The studies were also read and interpreted by the same gastroenterologists with expertise in gastrointestinal motility.
Acute cellular rejection was reviewed and graded by a trained pathologist according to International Society for Heart and Lung Transplantation (ISHLT) guidelines.
Nissen fundoplication procedures were performed at the discretion of transplant physicians. Various criteria included: symptomatic reflux and GERD as diagnosed by gastric studies using standardized conventions; and recurrent infections or rejection episodes suspected to be caused by reflux [14]. Fundoplication was not performed for single episodes of rejection or patients with hypertensive LES.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Bioethics Committee of the University of Louisville (IRB# 14.1171).
| Results | ▴Top |
The overall survival at 1 year was 92% for the included patients (54 out of 60) during the study timeframe. There were several abnormal findings of upper gastrointestinal testing in post-transplant patients. Notably, 37 patients (62%) had abnormal impedance testing, 50 patients (83%) had abnormal HRM results, and 22 patients (37%) had abnormal pH test results (Table 1). All six patients who died within 1 year of transplant had an abnormal impedance study, five had an abnormal HRM study, and three had an abnormal pH study.
 Click to view | Table 1. Total Number and Percent of Abnormal Post-Transplant Gastrointestinal Studies |
There was notable transplant rejection associated with abnormal esophageal motility testing following transplantation. All patients with acute cellular rejection were minimal rejection (A1) based on histopathology. There were 37 patients that had abnormal impedance test results and of those 25 patients (67%) developed rejection. Fifty patients had abnormal post-transplant HRM studies, 33 (66%) had an acute cellular rejection episode. Twenty-two patients had abnormal pH results, with 14 (63%) having an acute cellular rejection. Of the 60 transplanted patients, seven developed BOS (11%), and of these two had an abnormal impedance study, five had an abnormal HRM study, and one had an abnormal pH study (Table 2). From the study, 41 of the 60 patients (68%) had a rejection diagnosis.
 Click to view | Table 2. Mortality and Rejection Rates Associated With Abnormal Post-Transplant Gastrointestinal Studies |
Table 3 shows the distribution of the three predictors for those with and without rejection diagnoses, along with odds ratios and 95% confidence intervals from the logistic regression model. There was no relationship in the rates of abnormality in the predictors, and none were strongly related to a rejection diagnosis.
 Click to view | Table 3. Odds Ratio (OR) for Abnormal Motility Studies and Acute Cellular Rejection With Confidence Interval (CI) |
Of the 60 transplanted patients, six patients died. Median time from transplant to death was 10.3 ± 8.3 months (range 6.5 - 10.5 months). Rejection was found in 41 of all transplanted patients (68%), with 38 (93%) having resolution at the end of the study timeframe.
The management of the patients with acute cellular rejection and abnormal pH-impedance or HRM studies varied. Eleven patients received a Nissen fundoplication procedure postoperatively; and, of those, seven had an episode of acute cellular rejection as well as an abnormal impedance study. Interestingly, only four of the 25 patients with an acute cellular rejection and an abnormal impedance study received a fundoplication. Only two of the 14 patients with a history of acute cellular rejection and abnormal pH studies received fundoplication; five of the 11 patients with Nissen fundoplication had an abnormal pH study. Four of these five patients also had an abnormal HRM study. Three out of the 33 patients with abnormal HRM studies and rejection received Botox injections, all other patients received medical management.
| Discussion | ▴Top |
Several abnormalities of upper gut motility are seen in recipients undergoing post-transplant surveillance. Most post-transplant patients who underwent further gastrointestinal evaluation were found to have abnormal pH, impedance, standard manometry, or HRM results. This is clinically important, as most lung transplant recipients with abnormal results experienced some form of documented rejection. A high percentage of the patients studied with abnormal studies developed acute cellular rejection, though no notable association could be found (Table 3). Based on the findings of this study, upper gastrointestinal testing may prove to be useful in post-lung transplant surveillance protocols. Screening for upper gut dysmotility disorders and developing a formalized screening process may impact patient morbidity and mortality in lung transplant recipients. This might include, in the future, measurements of gastric emptying and cutaneous electrogastrography perhaps combined with gastro-pyloro-duodenal measures such as esophageal and pyloric functional luminal images probes (FLIP). In addition, new esophageal measures, such as those involving mucosal impedance of the esophagus, may reveal further associations with lung transplant rejection.
GERD is a well-known contributor to morbidity and health care-related costs in the USA. It effects upwards of 20% of the population [15]. Screening for GERD and gastroparesis is important in patients evaluated for and undergoing lung transplantation as they have increased associations with BOS and rejection [10, 16]. As noted in our data and in previous studies, there is substantial incidence of GERD and gastroparesis in patients pre- and post-lung transplantation [17]. Our data did not corroborate the association between esophageal motility disorders, by current standardized testing, and rejection episodes. This is important in understanding the presumed development of BOS and acute cellular rejection in relation to GERD and abnormal motility. Since the 1970s there has been an observational relationship between reflux disease and the development of pulmonary fibrosis [18]. Proposed mechanisms include incompetence of the LES, gastric dilatation, abnormal gastric pressure, increased gastric acid secretion, increased trans-diaphragmatic pressure with coughing and wheezing with lung disease, and elevated abdominal pressure with coughing. Chronic lung disease itself may also increase aspiration by disruption of the LES due to alterations of the chest wall and flattening of the diaphragm. LES barrier pressure may also be lowered with GERD, resulting in inflammatory cytokine release and injury of the vagal nerve, resulting in a recurrent cycle of reflux and aspiration [19]. By recognizing and treating the patients with reflux and gastroparesis before transplantation, there may be an opportunity to reduced BOS incidence and possibly improve survival.
Reflux and microaspiration have also been noted as substantial factors in the development of BOS post-transplant [20]. The presumed mechanism has not been completely established, but theorized pathways include impaired cough reflexes and mucociliary clearance. Studies have shown that clearance may be as low as 15% of normal function in transplanted patients [21]. Vagal nerve injury related to surgery, infection, and effects of immunosuppressive drugs has also been presumed cause of gastroparesis and reflux leading to increased risk for aspiration pneumonitis and development of rejection [22]. Improved outcomes would be expected by diagnosing and treating motility and reflux early in the post-transplant patient.
Our study demonstrates the prevalence of motility dysfunction in post-transplant patients, and the importance of screening and surveillance of reflux and in this population [15]. Through a review of the literature and various studies, we suggest methodology by which lung transplants should be screened and then monitored for abnormal esophageal function and gastroparesis. Possible newer technologies that can be used in this patient population are mentioned earlier in this discussion. Figure 1 details an algorithm, as well as basic guidelines in management once diagnosed [16, 17, 23-28]. In keeping with previously published procedures on the management of candidates for lung transplant, formal esophagogastroduodenoscopy (EGD), pH impedance, and HRM were performed prior to transplantation. Screening and treating patients for gastroparesis prior to transplantation in order to reduce the risk of BOS and rejection should be considered [17, 23]. From previous studies, it appears patients may benefit from interval testing post-transplant at 3, 6, and 12 months, and then at times of concern for BOS or acute cellular rejection to determine possible interventions to prevent recurrence [17, 23-28]. HRM is an incredibly important study in these populations, as it allows for the determination of which patients with reflux would potentially benefit from fundoplication [27]. Further investigations with larger patient populations and more formalized surveillance protocols are necessary to establish the relationship between upper gastrointestinal dysmotility and rejection in lung transplant recipients. As mentioned above, other measures of foregut motility, including newer esophageal, gastric, and duodenal measures, may be helpful. With this process, the goal would be to reduce lung transplant health costs by preventing hospitalizations and treatment for BOS, with resultant improvement in mortality.
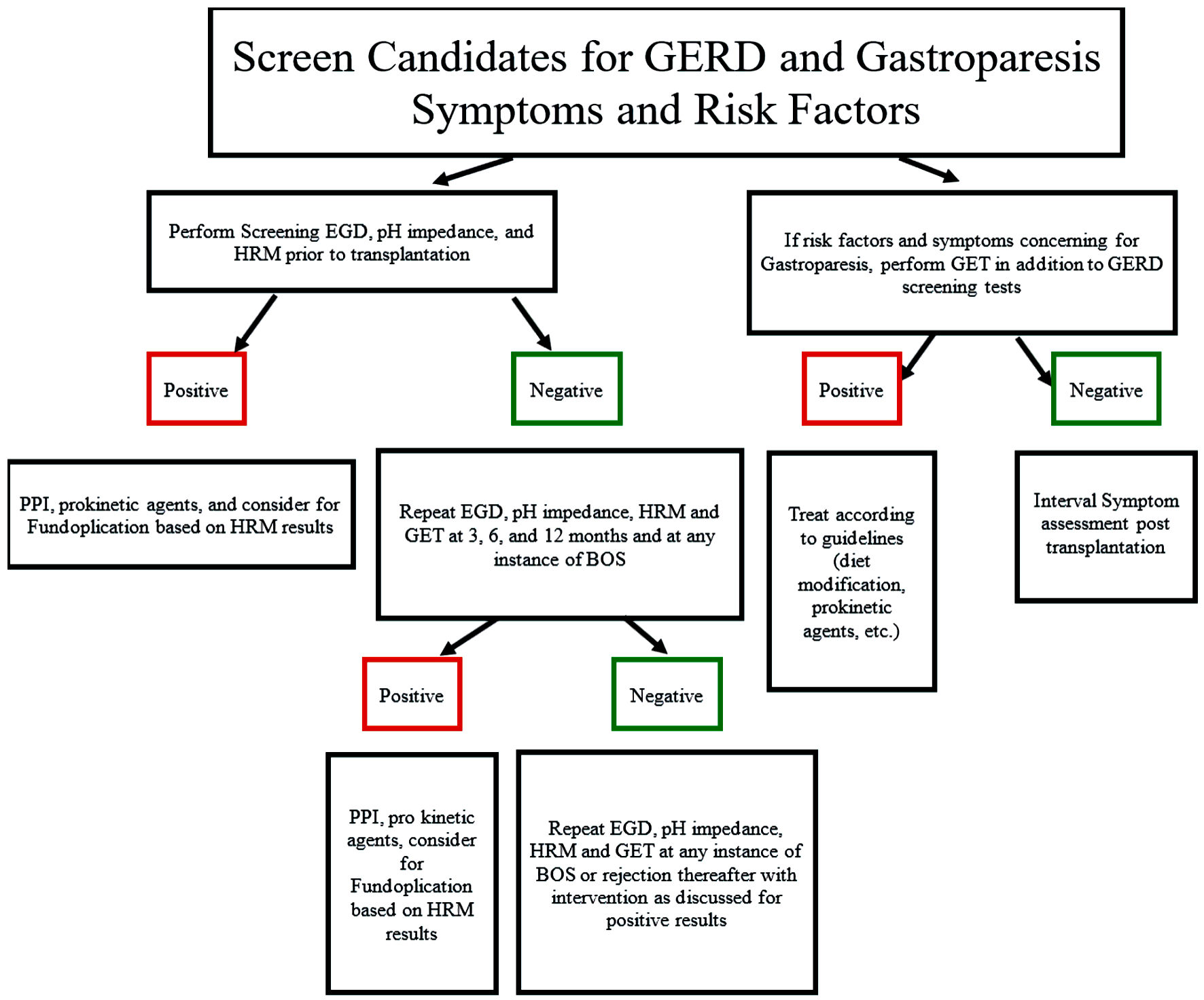 Click for large image | Figure 1. Algorithm for screening, monitoring, and treatment of lung transplant recipients for reflux and gastroparesis. GERD: gastroesophageal reflux disease; EGD: esophagogastroduodenoscopy; HRM: high-resolution manometry; BOS: bronchitis obliterans syndrome. |
Abnormalities of motility may be a major predisposing factor for rejection post-transplantation. A recent study by Blackett et al investigated the relationship between the development of gastroparesis post-transplantation and the development of CLAD. This study was particularly interesting, as there was an increase in GERD in patients that developed gastroparesis after transplant, but GERD itself was not associated with increased CLAD [29]. The study itself had several limitations, but the association between dysmotility and CLAD requires further investigation and more stringent post-transplant monitoring and evaluation for these complications.
Several patients in our study were found to have abnormal studies but did not receive surgical intervention. Several studies have been dedicated to linking acid reflux with rejection; however, many of our had normal pH studies. Notably, these patients had abnormal impedance or abnormal HRM and developed rejection. Management and treatment of these patients is of great interest [30, 31]. There has also been data demonstrating that symptoms alone are not sufficient to capture at-risk patients for esophageal motility disorders [32]. Further research into non-acid reflux and abnormal esophageal motility without acid reflux, perhaps with gastric and/or pyloric measures and its association with lung rejection may be warranted.
It is also notable that formal criteria have not been universally established for performing fundoplication in lung transplant patients. Recent studies have demonstrated discrepancy in post-transplant esophageal motility function. One recent retrospective cohort study demonstrated increased incidence of jackhammer esophagus after lung transplantation (15 out 57 patients) [33]. Yet, another retrospective cohort study found increased esophageal contractility amongst 76 patients studied; but 15 patients developed jackhammer esophagus post-transplant and patients with GERD had worse forced expiratory volume over 1 s (FEV1) compared to patients without GERD [34]. Establishing this is essential, especially given the movement toward earlier and more aggressive treatment [28]. Determining standardized variables and methods for establishing candidates for fundoplication is paramount to management as performing unnecessary or potentially harmful procedures without clear benefit is not desirable.
The limiting factors of our study are the retrospective nature and population size. No abnormal motility study was found to have an association with acute cellular rejection, but this may be limited by the number of patients in our study as well limits of standardized practices. Future prospective studies with larger patient populations would improve the ability to determine optimal timing of reflux and gastroparesis studies to optimize screening times and determine when post-transplantation reflux and BOS events most commonly develop. Our study was further limited in that it did not account for demographic disparity and, quite notably, 56 of the 60 transplant patients were white. Confounding variables that could result in lung injury and damage apart from acute cellular rejection and BOS were not accounted in this study secondary to a lack of consistent and standardized documentation complicated by the use of various medical documentation forms (e.g., more than one electronic medical record (EMR) and use of paper charts).The hope is that with more frequent and consistent testing, characterization of reflux and gastroparesis post-transplant will allow for better understanding of the relationship between these disease processes and acute cellular rejection, thereby providing future direction in diagnosis and prevention of this deadly complication.
Conclusions
Acute cellular rejection is a leading cause of morbidity and mortality amongst lung transplant recipients. Being able to reduce these events is paramount. It is also known that acute cellular rejection can develop at any time post-transplantation. This study shows the high number of patients that have abnormal motility and reflux, which has been linked to acute cellular rejection, but did not find clear association between standard esophageal dysmotility and rejection episodes. Many of these patients had acute cellular rejections and abnormal esophageal studies. Consistent and standardized expanded motility and reflux and other related upper gut studies pre- and post-transplant to screen and diagnose patients at risk for acute cellular rejection secondary to dysmotility should be determined.
Acknowledgments
The authors would like to thank the staff of the GI Motility Laboratory at Jewish Hospital/University of Louisville Health for assistance with the motility studies. They would like to thank Prateek Mathur for review of the manuscript, and Catherine McBride for assistance with manuscript preparation. They also would like to thank Michael Daniels for statistical review and help with data review.
Financial Disclosure
None to declare.
Conflict of Interest
Dr. Abell: main funding: NIH GpCRC; investigator: Censa, Cindome, Vanda, Allergan, Neurogastrix; consultant: Censa, Nuvaira, Takeda, Medtronic; speaker: Takeda, Medtronic; reviewer: UpToDate; GES editor: Neuromodulation, Wikistim; ADEPT-GI: IP for autonomic/enteric and bioelectric diagnosis and therapies; Gastric Dysrhythmias to NIH.
Informed Consent
This study had IRB approval from the University of Louisville. The informed consent is not applicable.
Author Contributions
Jordan Burlen, Suma Chennubhotla, and Shifat Ahmed: data analysis, manuscript preparation, and final review. Sarah Landes and Abigail M. Stocker: data acquisition, and final review. Allan Ramirez and Thomas L. Abell: data acquisition, manuscript preparation, and final review.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Tobin RW, Pope CE, 2nd, Pellegrini CA, Emond MJ, Sillery J, Raghu G. Increased prevalence of gastroesophageal reflux in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1998;158(6):1804-1808.
doi pubmed - Gualdoni J, Ritzenthaler J, Burlen J, Stocker A, Abell T, Roman J, Nunley DR. Gastroesophageal Reflux and Microaspiration in Lung Transplant Recipients: The Utility of a Single Esophageal Manometry and pH Probe Monitoring Study. Transplant Proc. 2020;52(3):977-981.
doi pubmed - Zhang CYK, Ahmed M, Huszti E, Levy L, Hunter SE, Boonstra KM, Moshkelgosha S, et al. Bronchoalveolar bile acid and inflammatory markers to identify high-risk lung transplant recipients with reflux and microaspiration. J Heart Lung Transplant. 2020;39(9):934-944.
doi pubmed - Fisichella PM, Davis CS, Lundberg PW, Lowery E, Burnham EL, Alex CG, Ramirez L, et al. The protective role of laparoscopic antireflux surgery against aspiration of pepsin after lung transplantation. Surgery. 2011;150(4):598-606.
doi pubmed - Biswas Roy S, Elnahas S, Serrone R, Haworth C, Olson MT, Kang P, Smith MA, et al. Early fundoplication is associated with slower decline in lung function after lung transplantation in patients with gastroesophageal reflux disease. J Thorac Cardiovasc Surg. 2018;155(6):2762-2771.e2761.
doi pubmed - Sundaresan S, Trulock EP, Mohanakumar T, Cooper JD, Patterson GA. Prevalence and outcome of bronchiolitis obliterans syndrome after lung transplantation. Washington University Lung Transplant Group. Ann Thorac Surg. 1995;60(5):1341-1346; discussion 1346-1347.
doi - Hofstetter E, Henig NR, Kappeler D. Prevalence of bronchiolitis obliterans syndrome (BOS) following allogeneic hematopoietic stem cell transplant (alloHSCT) in the USA. Europe and Japan. Blood. 2019;134(Supplement_1):5678-5678.
doi - Cooper JD, Patterson GA, Trulock EP. Results of single and bilateral lung transplantation in 131 consecutive recipients. Washington University Lung Transplant Group. J Thorac Cardiovasc Surg. 1994;107(2):460-470; discussion 470-461.
doi - Valentine VG, Robbins RC, Berry GJ, Patel HR, Reichenspurner H, Reitz BA, Theodore J. Actuarial survival of heart-lung and bilateral sequential lung transplant recipients with obliterative bronchiolitis. J Heart Lung Transplant. 1996;15(4):371-383.
- Raviv Y, D'Ovidio F, Pierre A, Chaparro C, Freeman M, Keshavjee S, Singer LG. Prevalence of gastroparesis before and after lung transplantation and its association with lung allograft outcomes. Clin Transplant. 2012;26(1):133-142.
doi pubmed - Cantu E, 3rd, Appel JZ, 3rd, Hartwig MG, Woreta H, Green C, Messier R, Palmer SM, et al. J. Maxwell Chamberlain Memorial Paper. Early fundoplication prevents chronic allograft dysfunction in patients with gastroesophageal reflux disease. Ann Thorac Surg. 2004;78(4):1142-1151; discussion 1142-1151.
doi pubmed - Davidson JR, Franklin D, Kumar S, Mohammadi B, Dawas K, Eaton S, Curry J, et al. Fundoplication to preserve allograft function after lung transplant: Systematic review and meta-analysis. J Thorac Cardiovasc Surg. 2020;160(3):858-866.
doi pubmed - Berkowitz N, Schulman LL, McGregor C, Markowitz D. Gastroparesis after lung transplantation. Potential role in postoperative respiratory complications. Chest. 1995;108(6):1602-1607.
doi pubmed - Cho YK. How to Interpret Esophageal Impedance pH Monitoring. J Neurogastroenterol Motil. 2010;16(3):327-330.
doi pubmed - Gawron AJ, Hirano I. Advances in diagnostic testing for gastroesophageal reflux disease. World J Gastroenterol. 2010;16(30):3750-3756.
doi pubmed - D'Ovidio F, Singer LG, Hadjiliadis D, Pierre A, Waddell TK, de Perrot M, Hutcheon M, et al. Prevalence of gastroesophageal reflux in end-stage lung disease candidates for lung transplant. Ann Thorac Surg. 2005;80(4):1254-1260.
doi pubmed - Robertson AG, Ward C, Pearson JP, Corris PA, Dark JH, Griffin SM. Lung transplantation, gastroesophageal reflux, and fundoplication. Ann Thorac Surg. 2010;89(2):653-660.
doi pubmed - Mays EE, Dubois JJ, Hamilton GB. Pulmonary fibrosis associated with tracheobronchial aspiration. A study of the frequency of hiatal hernia and gastroesophageal reflux in interstitial pulmonary fibrosis of obscure etiology. Chest. 1976;69(4):512-515.
doi pubmed - Ledson MJ, Tran J, Walshaw MJ. Prevalence and mechanisms of gastro-oesophageal reflux in adult cystic fibrosis patients. J R Soc Med. 1998;91(1):7-9.
doi pubmed - Sweet MP, Patti MG, Hoopes C, Hays SR, Golden JA. Gastro-oesophageal reflux and aspiration in patients with advanced lung disease. Thorax. 2009;64(2):167-173.
doi pubmed - Kirk AJ, Colquhoun IW, Corris PA, Hilton CJ, Dark JH. Impaired gastrointestinal motility in pulmonary transplantation. Lancet. 1990;336(8717):752.
doi - Castor JM, Wood RK, Muir AJ, Palmer SM, Shimpi RA. Gastroesophageal reflux and altered motility in lung transplant rejection. Neurogastroenterol Motil. 2010;22(8):841-850.
doi pubmed - D'Ovidio F, Mura M, Ridsdale R, Takahashi H, Waddell TK, Hutcheon M, Hadjiliadis D, et al. The effect of reflux and bile acid aspiration on the lung allograft and its surfactant and innate immunity molecules SP-A and SP-D. Am J Transplant. 2006;6(8):1930-1938.
doi pubmed - Davis RD Jr., Lau CL, Eubanks S, Messier RH, Hadjiliadis D, Steele MP, Palmer SM. Improved lung allograft function after fundoplication in patients with gastroesophageal reflux disease undergoing lung transplantation. The Journal of Thoracic and Cardiovascular Surgery. 2003;125(3):533-542.
doi pubmed - Robertson AG, Ward C, Pearson JP, Small T, Lordan J, Fisher AJ, Bredenoord AJ, et al. Longitudinal changes in gastro-oesophageal reflux from 3 months to 6 months after lung transplantation. Thorax. 2009;64(11):1005-1007.
doi pubmed - Mertens V, Dupont L, Sifrim D. Relevance of GERD in lung transplant patients. Curr Gastroenterol Rep. 2010;12(3):160-166.
doi pubmed - Hartwig MG, Anderson DJ, Onaitis MW, Reddy S, Snyder LD, Lin SS, Davis RD. Fundoplication after lung transplantation prevents the allograft dysfunction associated with reflux. Ann Thorac Surg. 2011;92(2):462-468; discussion; 468-469.
doi pubmed - Patti MG, Vela MF, Odell DD, Richter JE, Fisichella PM, Vaezi MF. The intersection of GERD, aspiration, and lung transplantation. J Laparoendosc Adv Surg Tech A. 2016;26(7):501-505.
doi pubmed - Blackett JW, Benvenuto L, Leiva-Juarez MM, D'Ovidio F, Arcasoy S, Jodorkovsky D. Risk factors and outcomes for gastroparesis after lung transplantation. Dig Dis Sci. 2022;67(6):2385-2394.
doi pubmed - Blondeau K, Mertens V, Vanaudenaerde BA, Verleden GM, Van Raemdonck DE, Sifrim D, Dupont LJ. Gastro-oesophageal reflux and gastric aspiration in lung transplant patients with or without chronic rejection. Eur Respir J. 2008;31(4):707-713.
doi pubmed - Lo WK, Goldberg HJ, Chan WW. Su1059 - concurrent non-acid reflux is associated with chronic rejection in lung transplant patients with acid reflux on pre-transplant testing. Gastroenterology. 2018;154(6):S470-S471.
doi - Posner S, Zheng J, Wood RK, Shimpi RA, Hartwig MG, Chow SC, Leiman DA. Gastroesophageal reflux symptoms are not sufficient to guide esophageal function testing in lung transplant candidates. Dis Esophagus. 2018;31(5):dox157.
doi - Cangemi DJ, Flanagan R, Bailey A, Staller K, Kuo B. Jackhammer Esophagus After Lung Transplantation: Results of a Retrospective Multicenter Study. J Clin Gastroenterol. 2020;54(4):322-326.
doi pubmed - Posner S, Finn RT, Shimpi RA, Wood RK, Fisher D, Hartwig MG, Klapper J, et al. Esophageal contractility increases and gastroesophageal reflux does not worsen after lung transplantation. Dis Esophagus. 2019;32(10):1-8.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


