| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website http://www.gastrores.org |
Case Report
Volume 11, Number 3, June 2018, pages 231-234
Renal Cell Carcinoma Presenting as an Ampullary Mass: A Case Report and Review of Literature
Syed Hasana, b, Zubair Khana, d, Mohammad Saud Khana, Umar Darra, Toseef Javaida, Raheel Ahmedc, Ali Nawrasa, b
aDepartment of Internal Medicine, University of Toledo Medical Center, Toledo, OH, USA
bDivision of Gastroenterology, University of Toledo, Toledo, OH, USA
cDepartment of Pathology, University of Toledo, Toledo, OH, USA
dCorresponding Author: Zubair Khan, Department of Internal Medicine, University of Toledo, 3000 Arlington Avenue, Toledo, OH 43614, USA
Manuscript submitted February 3, 2018, accepted February 27, 2018
Short title: RCC as Ampullary Mass
doi: https://doi.org/10.14740/gr981w
| Abstract | ▴Top |
We present a case of a 60-year-old female patient who has significant medical history of renal cell carcinoma diagnosed 2 years back and had undergone right nephrectomy and chemotherapy. She presented to the hospital with complaints of abdominal pain and jaundice of 2 weeks duration and was found to have periampullary mass lesion causing compression of distal common bile duct on imaging with computed tomography of abdomen. Endoscopic retrograde cholangiography and endoscopic ultrasound showed ampullary mass lesion causing biliary obstruction along with abdominal lymphadenopathy. A temporary plastic stent was placed to relieve obstruction. Fine needle aspiration cytology of the periampullary mass along with immunohistochemical staining confirmed the diagnosis of metastatic renal cell carcinoma.
Keywords: Renal cell carcinoma; Ampullary mass; Obstructive jaundice
| Introduction | ▴Top |
Renal cell carcinoma (RCC) is the most common renal malignancy and second most common malignancy of urological tract [1]. It accounts for approximately 85-90% cases of malignant renal neoplasm [2]. RCC comprises of heterogeneous group of cancers that originate from renal tubular epithelial cells [3]. Each of these cancers has varied histology, is caused by mutations in different genes and responds differently to treatment. Major histopathological subtypes include clear cell, papillary, chromophobe, collecting duct carcinoma, medullary carcinoma and unclassified categories [4]. Clear cell carcinoma is the most common subtype making up to 75% of cases of RCC [3]. RCC is known to metastasize in unpredictable manner [2]. However, metastasis to the ampulla of Vater is extremely rare with very few cases reported in literature. These patients usually present with symptoms of obstructive jaundice, melena, and malabsorption [5-7]. Treatment options include surgery with pancreatoduodenectomy and nephrectomy followed by adjuvant systemic chemotherapy/immunotherapy [8].
| Case Report | ▴Top |
A 60-year-old female patient presented with 2 weeks history of abdominal pain, fatigue, jaundice along with nausea and vomiting. She has a significant medical history of RCC (T2N0M0) diagnosed 2 years back and had undergone right nephrectomy and chemotherapy. Imaging with computed tomography (CT) of abdomen was done which showed periampullary mass lesion compressing the distal end of common bile duct (CBD) leading to dilatation of proximal CBD and intrahepatic biliary channels. She was taken for endoscopic retrograde cholangiopancreatography (ERCP) and endoscopic ultrasound (EUS), which revealed a large ampullary mass causing biliary obstruction with multiple large subcarinal, celiac, and paraduodenal lymph nodes (largest size, 42 × 36 mm). Fine needle aspirates (FNA) were taken from ampullary mass and subcarinal lymph nodes. The ampulla was dilated, endoscopic sphincterotomy was performed and a temporary plastic biliary stent was placed. FNA cytology from ampullary mass and lymph nodes was consistent with metastatic RCC and this was confirmed with immunohistochemical staining (Fig. 1a, b). Patient reported improvement in the symptoms of nausea, vomiting and pain. Liver function tests were repeated next day and showed improvement with decreasing total bilirubin (from 7.1 to 4.2 mg/dL) and alkaline phosphatase (540 - 260 U/L). A positron emission tomography (PET) scan was planned to look for other foci of metastasis but patient elected to follow up with her own oncologist outside of our institution and was lost to us for follow-up.
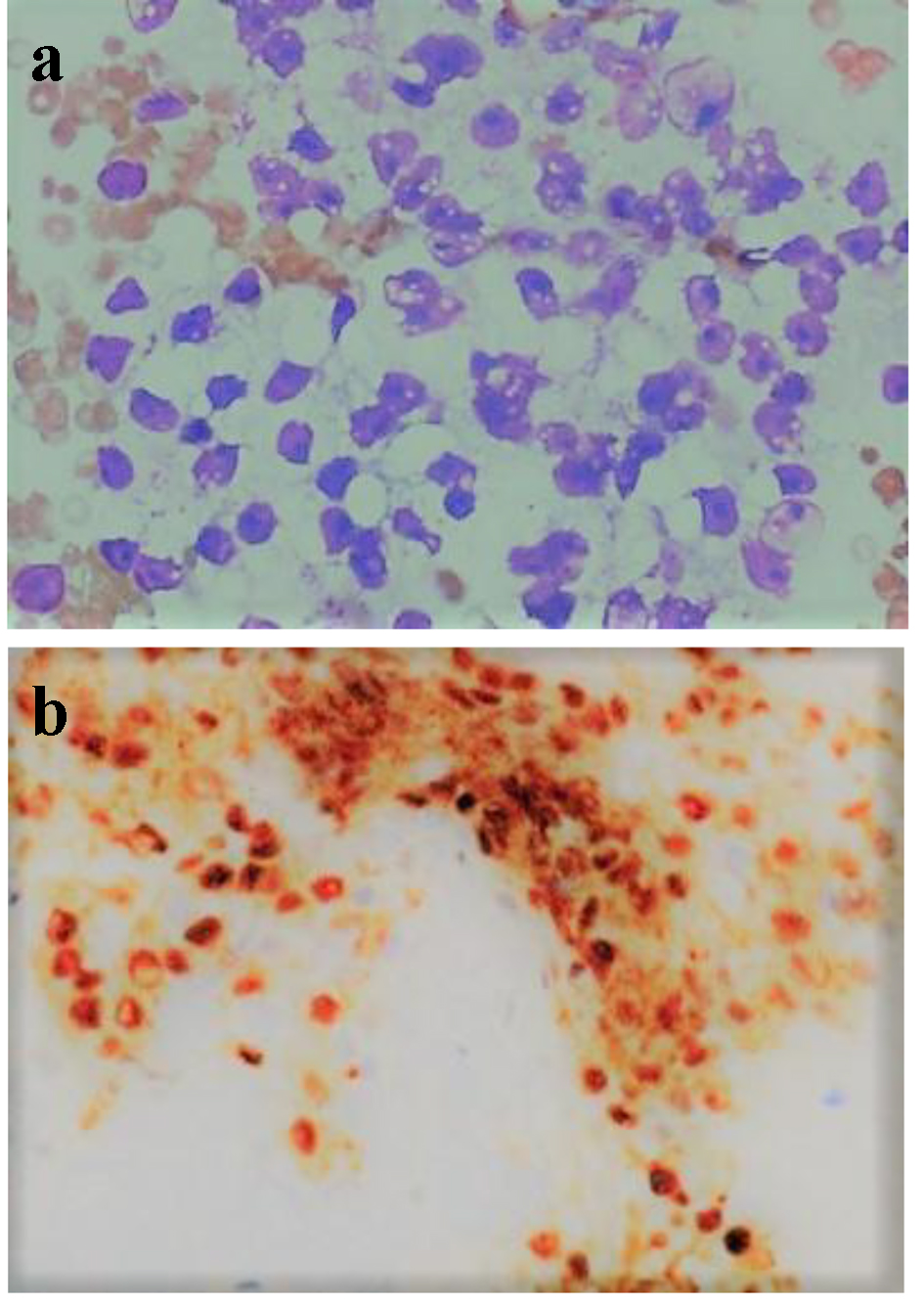 Click for large image | Figure 1. (a) FNA cytology of ampullary mass (in higher magnification, × 400) showing tumor cells with marked nuclear pleomorphism, prominent nucleoli, and overt vacuolization to the cytoplasm. (b) Immunohistochemistry showing diffuse nuclear immunoreactivity for PAX 8. |
| Discussion | ▴Top |
RCC is the sixth most common cancer among males and 10th most common among females in United States [1]. It accounts for approximately 64,000 new cases and 14,000 deaths each year in United States [1]. It is more common in males as compared to females (ratio of 2:1) and occurs predominantly in sixth to eighth decade of life with a median age of 64 years [1]. Established risk factors for RCC include obesity, hypertension and cigarette smoking. Besides these chronic kidney disease, acquired renal cystic disease, hemodialysis, and renal transplant have been associated with RCC [9]. RCC is anatomically staged I-IV, as per American Joint Committee on Cancer (AJCC) tumor node metastasis (TNM) classification system. The stage of disease at the time of presentation is correlated with the prognosis and survival [10, 11]. The 5-year survival of patients with organ-confined disease (stage I and II RCC) without invasion of urinary collecting system is good and ranges from 80% to 95% [3, 10]. Invasion of urinary collecting system in stage I and II RCC is associated with relatively poorer prognosis with 5-year survival of around 60% [10, 12]. For metastatic disease (stage IV RCC), the prognosis is extremely poor with 5-year survival rate of less than 10% and median survival of 10 months [10, 13]. Approximately two-thirds of patients have localized disease at the time of presentation and surgical treatment (partial or radical nephrectomy) with curative intent is the gold standard in these patients [14]. The choice of partial over radical nephrectomy is determined by tumor location and stage.
RCC is an aggressive tumor with propensity for metastatic spread. It has been associated with metastasis to unusual sites occurring both synchronously or metachronously [2]. It has been estimated that 20-30% of patients including those who have undergone nephrectomy with curative intent will develop recurrence and out of these 50% will relapse distantly [15]. Most of the recurrences are within 3 - 5 years of surgery but delayed recurrences even after decades have been reported [13, 15, 16]. The longer the recurrence free time from surgery the more likelihood is of a true cure. The most common pathway of metastatic spread in RCC is hematogenous and the likely sites of metastasis include lung, liver, lymph nodes, bones, and adrenal glands [2]. Metastasis to gastrointestinal tract is unusual with large intestine, pancreas and peritoneum being commoner sites. Metastasis to ampulla of Vater is extremely rare and only few cases have been reported in literature [8]. On review of literature, we found only 11 reported cases of RCC metastazing to ampulla of Vater. To our knowledge, this is the 12th case of RCC with metastasis to the ampulla (Table 1 [5-8, 17-21]). Of these cases, seven were males and five were females. The median age was 65 years. Of the 12 patients, seven presented with the symptoms of gastrointestinal bleeding, while rest four presented with jaundice. All the patients except one were known to have RCC and had undergone nephrectomy prior to being diagnosed with metastatic ampullary cancer. The median interval from nephrectomy to detection of ampullary metastasis was 8.36 years, with one of the case not reporting the interval. All but one patient had unilateral renal cell cancer, with one having a recurrence in the contralateral kidney prior to presenting with ampullary metastasis. Eight out of 12 patients were reported to have undergone surgery of which one patient died post-operatively from a pulmonary embolism, two of the patients died of metastatic cancer, one patient’s outcome was not reported and our patient was still undergoing treatment for metastatic diseases but more detailed information was not available to us.
 Click to view | Table 1. Review of Literature [5-8, 17-21] |
Patients who have metastatic disease at the time of presentation are treated with cytoreductive surgery followed by systemic chemotherapy/immunotherapy [22]. Metastatic RCCs are resistant to traditional chemotherapeutic drugs. Interleukin-2 and interferon alpha, which have traditionally been utilized as adjuvant therapy for many years, have low response rates [23]. Recently with advances in treatment options targeted therapy with VEGFR inhibitors (sorafenib, sunitinib, bevacizumab, pazopanib, and axitanib) and mTOR inhibitors (everolimus and temsirolimus) has been approved [23]. These drugs have better response rate and have shown to increase overall survival in metastatic RCC. Metastatic disease has extremely poor prognosis with 5-year survival rate of less than 10% and median survival of 10 months [10, 13]. However ampullary metastasis, by early observation of case reports, appears to have relatively better prognosis as it is amenable to surgical resection. According to Haidong et al [8], the prognosis for ampullary metastasis is relatively good, due to the availability of surgical options, with pancreatoduodenectomy and nephrectomy being the therapy of choice. However, due to the scarcity of cases, the outcome of this condition is not yet fully established and prognosis is not clearly defined.
Author Contributions
Zubair Khan contributed in writing the case report and major parts of discussion. Mohammad Saud Khan and Syed Hasan assisted in writing the manuscript. Umar darr and Toseef Javaid assisted in the literature review. Ali Nawras supervised and reviewed the entire article.
| References | ▴Top |
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7-30.
doi pubmed - Motzer RJ, Bander NH, Nanus DM. Renal-cell carcinoma. N Engl J Med. 1996;335(12):865-875.
doi pubmed - Hsieh JJ, Purdue MP, Signoretti S, Swanton C, Albiges L, Schmidinger M, Heng DY, et al. Renal cell carcinoma. Nat Rev Dis Primers. 2017;3:17009.
doi pubmed - Kovacs G, Akhtar M, Beckwith BJ, Bugert P, Cooper CS, Delahunt B, Eble JN, et al. The Heidelberg classification of renal cell tumours. J Pathol. 1997;183(2):131-133.
doi - Bolkier M, Ginesin Y, Moskovitz B, Munichor M, Levin DR. Obstructive jaundice caused by metastatic renal cell carcinoma. Eur Urol. 1991;19(1):87-88.
doi pubmed - Janzen RM, Ramj AS, Flint JD, Scudamore CH, Yoshida EM. Obscure gastrointestinal bleeding from an ampullary tumour in a patient with a remote history of renal cell carcinoma: a diagnostic conundrum. Can J Gastroenterol. 1998;12(1):75-78.
doi pubmed - McKenna JI, Kozarek RA. Metastatic hypernephroma to the ampulla of Vater: an unusual cause of malabsorption diagnosed at endoscopic sphincterotomy. Am J Gastroenterol. 1989;84(1):81-83.
pubmed - Haidong W, Jianwei W, Guizhong L, Ning L, Feng H, Libo M. Ampullary tumor caused by metastatic renal cell carcinoma and literature review. Urol J. 2014;11(2):1504-1507.
pubmed - Lipworth L, Tarone RE, Lund L, McLaughlin JK. Epidemiologic characteristics and risk factors for renal cell cancer. Clin Epidemiol. 2009;1:33-43.
pubmed - Jonasch E, Gao J, Rathmell WK. Renal cell carcinoma. BMJ. 2014;349:g4797.
doi pubmed - Cairns P. Renal cell carcinoma. Cancer Biomark. 2010;9(1-6):461-473.
doi pubmed - Verhoest G, Avakian R, Bensalah K, Thuret R, Ficarra V, Artibani W, Tostain J, et al. Urinary collecting system invasion is an independent prognostic factor of organ confined renal cell carcinoma. J Urol. 2009;182(3):854-859.
doi pubmed - Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999;17(8):2530-2540.
doi pubmed - Campbell SC, Novick AC, Belldegrun A, Blute ML, Chow GK, Derweesh IH, Faraday MM, et al. Guideline for management of the clinical T1 renal mass. J Urol. 2009;182(4):1271-1279.
doi pubmed - Bukowski RM. Prognostic factors for survival in metastatic renal cell carcinoma: update 2008. Cancer. 2009;115(10 Suppl):2273-2281.
doi pubmed - Flanigan RC, Campbell SC, Clark JI, Picken MM. Metastatic renal cell carcinoma. Curr Treat Options Oncol. 2003;4(5):385-390.
doi pubmed - Karakatsanis A, Vezakis A, Fragulidis G, Staikou C, Carvounis EE, Polydorou A. Obstructive jaundice due to ampullary metastasis of renal cell carcinoma. World J Surg Oncol. 2013;11:262.
doi pubmed - Leslie KA, Tsao JI, Rossi RL, Braasch JW. Metastatic renal cell carcinoma to ampulla of Vater: an unusual lesion amenable to surgical resection. Surgery. 1996;119(3):349-351.
doi - Venu RP, Rolny P, Geenen JE, Hogan WJ, Komorowski RA, Ferstenberg R. Ampullary tumor caused by metastatic renal cell carcinoma. Dig Dis Sci. 1991;36(3):376-378.
doi pubmed - Robertson GS, Gertler SL. Late presentation of metastatic renal cell carcinoma as a bleeding ampullary mass. Gastrointest Endosc. 1990;36(3):304-306.
doi - Franssen B, Chan C, Ramirez-Del Val A, Llamas F, Lopez-Tello A. [Renal cell carcinoma metastatic to the duodenum and Vater ampulla: report of two cases]. Rev Gastroenterol Mex. 2011;76(4):375-379.
pubmed - Heng DY, Wells JC, Rini BI, Beuselinck B, Lee JL, Knox JJ, Bjarnason GA, et al. Cytoreductive nephrectomy in patients with synchronous metastases from renal cell carcinoma: results from the International Metastatic Renal Cell Carcinoma Database Consortium. Eur Urol. 2014;66(4):704-710.
doi pubmed - Choueiri TK, Motzer RJ. Systemic therapy for metastatic renal-cell carcinoma. N Engl J Med. 2017;376(4):354-366.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


