| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website http://www.gastrores.org |
Review
Volume 10, Number 2, April 2017, pages 65-69
IL-10 and IL-10 Receptor Mutations in Very Early Onset Inflammatory Bowel Disease
Lei Zhua, Tingting Shia, Chengdi Zhonga, Yingde Wanga, Michael Changb, Xiuli Liub, c
aDepartment of Gastroenterology and Hepatology, The First Affiliated Hospital of Dalian Medical University, Dalian City, China
bDepartment of Pathology, Immunology, and Laboratory Medicine, University of Florida, Gainesville, FL, USA
cCorresponding Author: Xiuli Liu, Department of Anatomic Pathology, Immunology, and Laboratory Medicine, University of Florida, Gainesville, FL 32610, USA
Manuscript accepted for publication December 15, 2016
Short title: IL-10 and IL-10 Receptor Mutations in VEO-IBD
doi: https://doi.org/10.14740/gr740w
- Abstract
- Introduction
- Physiological Function of IL-10 in the Gut
- Mutations in IL-10 and IL-10R in VEO-IBD
- Clinical Features of IL-10 or IL-10R Mutation-Associated VEO-IBD
- Conclusion
- References
| Abstract | ▴Top |
Very early onset inflammatory bowel disease (VEO-IBD) is a unique disease entity with a complex genetic susceptibility in affected patients. Next-generation gene sequencing techniques have revealed various monogenetic mutations contributing to the pathogenesis of VEO-IBD, including interleukin 10 (IL-10) and IL-10 receptor (IL-10R) mutations. In this article, we reviewed the features of and effective therapeutic options for VEO-IBD with IL-10 and/or IL-10R mutations. The IL-10 signal pathway inhibits the release of several key cytokines and thereby has a significant anti-inflammatory effect in the gastrointestinal tract. Mutations of the genes encoding IL-10 and/or IL-10R have been detected in VEO-IBD patients among myriad populations throughout the world. VEO-IBD patients with IL-10 or IL-10R mutations often present with repeated bouts of bloody diarrhea, marked weight loss, growth retardation, and recurrent perianal problems, including abscesses, fistulas, and significant fissures. Moreover, some patients may have folliculitis and present with pulmonary infections. While the therapeutic efficacy of immunosuppressants is typically poor in these patients, allogeneic hematopoietic stem cell transplantation (HSCT) has been reported to improve symptoms significantly. However, the long-term prognosis of VEO-IBD patients with IL-10 or IL-10R gene mutations treated with HSCT requires further exploration to verify the efficacy and safety of this treatment. We concluded that clinicians should recognize the clinical phenotype of VEO-IBD, as mutational analysis of the IL-10 pathway can support the diagnosis and prompt early treatment of this complicated disease.
Keywords: Interleukin-10; Interleukin-10 receptor; Gene mutation; Inflammatory bowel disease
| Introduction | ▴Top |
Inflammatory bowel disease (IBD) is a heterogeneous family of chronic gastrointestinal inflammatory disorders encompassing ulcerative colitis (UC), Crohn’s disease (CD), and indeterminate colitis. The etiology and pathogenesis of these disorders have yet to be entirely elucidated. Studies increasingly reveal that the chronic inflammation of the gut in IBD is triggered by a variety of environmental factors in genetically susceptible individuals. Very early onset IBD (VEO-IBD) is defined as IBD with a disease onset before 6 years of age. Compared with adolescent- and adult-onset IBD, in which exogenous environmental factors are thought to play a larger role, genetic susceptibility may have more of an influence on the pathogenesis of VEO-IBD [1]. Genome-wide association studies (GWASs) have discovered 201 candidate loci of probable genetic mutations related to IBD; the most widely reported mutated gene codes for nucleotide-binding oligomerization domain-containing protein 2 (NOD2) [2]. GWAS is useful in detecting common genetic variants, but is not suitable for identifying low-frequency monogenetic variants, which seem to be more relevant to VEO-IBD [3]. In contrast, genetic linkage analysis and exome gene sequencing can overcome the limitations of GWAS. Until now, at least 58 susceptible genes were implicated in the pathogenesis of VEO-IBD by new gene sequencing techniques [4]. Among these susceptible genes, interleukin-10 (IL-10) and IL-10 receptor (IL-10R) gene mutations have been extensively investigated. This article aimed to review the current literature on the mutations of IL-10 and IL-10R in VEO-IBD.
| Physiological Function of IL-10 in the Gut | ▴Top |
IL-10 is an important anti-inflammatory cytokine secreted by various cells, including monocytes, macrophages, T and B lymphocytes, dendritic cells, epithelial cells, and mast cells. IL-10 inhibits the release of tumor necrosis factor α [5] and thus is critical to the maintenance of immune homeostasis in gastrointestinal tract. The immune-modulating effect of IL-10 starts with its binding to IL-10R, which is a tetrameric receptor complex consisting of two α sub-units of IL-10 receptor 1 (IL-10R1), encoded by IL-10RA, and two β sub-units of IL-10 receptor 2 (IL-10R2), encoded by IL-10RB [6]. IL-10R1 only binds with IL-10, whereas the IL-10R2 sub-unit can bind with several other cytokines, such as IL-22, IL-26, IL-28 and IL-29 [7]. Once IL-10 binds its receptor, it activates Janus kinase 1 (JAK1) and tyrosine kinase 2 (Tyk2), leading to the phosphorylation of signal transducer and activator of transcription 3 (STAT-3), the activation of downstream target genes, and finally the expression of anti-inflammatory effectors [8]. The IL-10 and IL-10R signal pathway is briefly shown in Figure 1.
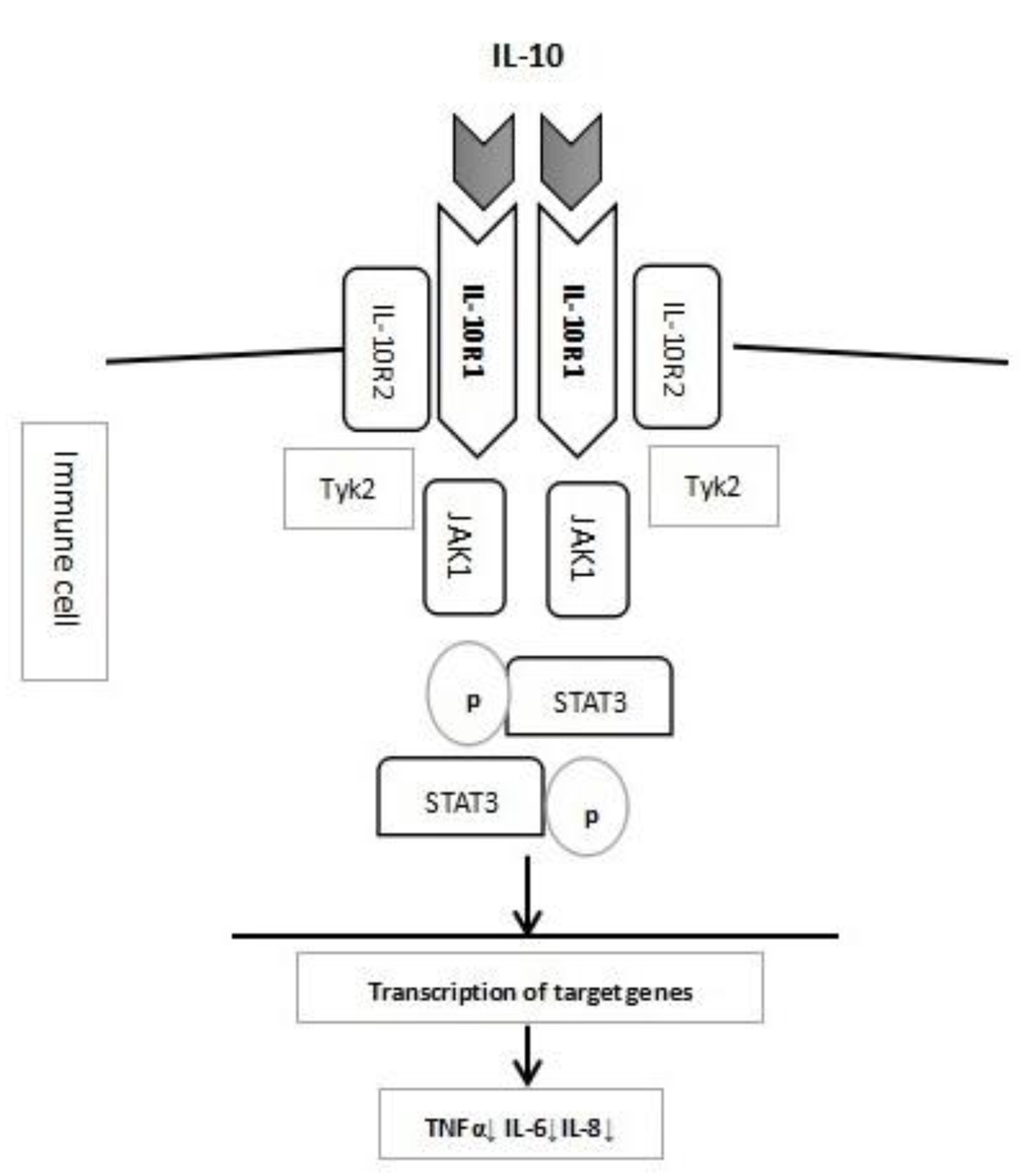 Click for large image | Figure 1. Diagram of IL-10 and IL-10R pathway. |
| Mutations in IL-10 and IL-10R in VEO-IBD | ▴Top |
Defects in the encoding regions of IL-10 and IL-10R genes lead to disturbances of the anti-inflammatory response. The dysfunction of this IL-10 and IL-10R signal pathway in humans can cause severe enterocolitis [9], as seen in some cases of VEO-IBD. Since the initial report in 2009 by Glocker et al [10], more than 60 cases of IL-10 or IL-10R gene mutated VEO-IBD have been documented in the literature. Most of the cases were reported in Europe, where IL-10R mutations were more predominant than IL-10 mutations. In 22 cases reported in East Asia, 21 cases had IL-10RA mutations, and only one case had an IL-10RB mutation, in contrast to European cases, in which the numbers of IL-10RA and IL-10RB mutated cases were somewhat more equivalent (Table 1). Cases of IL-10 and IL-10R mutated VEO-IBD in the literature are summarized in Table 1 [10-27].
 Click to view | Table 1. Cases of VEO-IBD with mutated IL-10, IL-10RA, or IL-10RB reported in the literature [10-27] |
Until now, most cases of IL-10 and/or IL-10R mutations have been scattered reports, and multi-center and global epidemiological data are lacking. Pediatric IBD patients (defined as younger than 16 years) account for approximately 20-25% of all IBD patients. About 5% of all IBD patients are less than 10 years old, and approximately 1% are less than 2 years of age [1]. Approximately 15% of pediatric IBD patients can be classified as VEO-IBD [27]. Although IL-10 and/or IL-10R mutations occur in VEO-IBD, the exact prevalence is not known. In small cohorts of VEO-IBD, the frequency of IL-10 and/or IL-10R varied significantly. For example, one report from the United Kingdom showed that five of 62 patients (8.1%) with IBD younger than 2 years old were verified to have IL-10 or IL-10R mutation [28]. One study from Germany reported that 16 of 66 (24.2%) IBD patients younger than 5 years old had IL-10 or IL-10R mutations [13]. In the United States, only six of 125 (4.8%) VEO-IBD patients showed IL-10 mutations [24]. Meanwhile, VEO-IBD cases from Asian countries tend to have higher rate of IL-10/IL-10R mutations. For example, one study from Korea reported that seven of 14 IBD patients (50%) diagnosed within 1 year of age had IL-10RA mutations [20]. The prevalence of IL-10R mutations was also strikingly high in VEO-IBD cases from China. According to a report by Xiao et al [27], 38.5% of VEO-IBD cases from China are positive for IL-10RA or IL-10RB mutations. This high frequency of IL-10 and/or IL-10R mutations in China is probably due to the small size of the cohort as there were only 13 VEO-IBD cases in this study. Regardless, the currently available data from case series suggest that the frequency of IL-10 and/or IL-10R gene mutations should not be low. Multi-center studies are needed to determine the frequency and role of IL-10 and/or IL-10R mutations in VEO-IBD and to accurately identify the clinical phenotype of IL-10 and/or IL-10R mutated VEO-IBD. Such information could potentially be used in the future to guide the screening of VEO-IBD patients with IL-10 and/or IL10-R mutations.
| Clinical Features of IL-10 or IL-10R Mutation-Associated VEO-IBD | ▴Top |
Compared with adult-onset IBD, the clinical manifestations of VEO-IBD are different. VEO-IBD has a more severe clinical course and is more resistant to immunosuppressive therapy. VEO-IBD patients with IL-10 or IL-10R mutations have even more complicated, severe, and intractable disease. These patients have repeated bouts of bloody diarrhea, marked weight loss, growth retardation, and recurrent perianal inflammation with abscesses, fistulas, and significant fissures [29]. Furthermore, folliculitis and refractory pneumonia are frequent complications in patients with IL-10RB mutations which interrupt the binding of IL-10R1 and IL-22, finally leading to disturbances in skin and lung epithelial immunity due to abnormal IL-22 signaling pathway [13, 25].
In addition to the severe and intractable nature of VEO-IBD with IL-10 or IL-10R mutations, this disease is also resistant to a variety of immunosuppressive therapies, including azathioprine, methotrexate, corticosteroid, and infliximab, either as single agent therapies or in combination. Treatment with the above agents usually results in no or only mild improvement of clinical manifestations. A few patients have had to undergo bowel resection and ileostomy or colostomy due to poor treatment efficacy and resistance to therapy [22]. Given the fact that IL-10 acts predominantly on hematopoietic and immune cells, allogeneic hematopoietic stem cell transplantation (HSCT) has been attempted as a curative therapy for VEO-IBD patients with IL-10 or IL-10R mutations [10, 13, 17, 19, 23, 25, 30]. The initial results appear to support the therapeutic role of HSCT in VEO-IBD patients with IL-10 or IL-10R mutations. However, experience is limited, as it was only used in a few patients with a relatively short follow-up period. HSCT remains a promising therapeutic modality, but additional studies are required to address its long-term safety and efficacy in VEO-IBD patients with IL-10 or IL-10R mutations.
| Conclusion | ▴Top |
VEO-IBD is a rare disease with a high probability of harboring monogenetic mutations, such as IL-10 or IL-10R mutations. It is important for clinicians to recognize the phenotype of IL-10 gene or IL-10R gene mutated VEO-IBD and prompt genetic testing to aid in the early diagnosis and treatment of these patients. In patients with IL-10 gene or IL-10R gene mutated VEO-IBD who are refractory to standard medical therapy, allogeneic HSCT may be an efficacious treatment option in a clinical trial setting.
Disclosure
None.
Abbreviations
CD: Crohn’s disease; GWAS: genome-wide association study; HSCT: hematopoietic stem cell transplantation; IBD: inflammatory bowel disease; IL: interleukin; NOD2: nucleotide-binding oligomerization domain-containing protein 2; STAT-3: signal transducer and activator of transcription 3; UC: ulcerative colitis; VEO-IBD: very early onset inflammatory bowel disease
| References | ▴Top |
- Snapper SB. Very-Early-Onset Inflammatory Bowel Disease. Gastroenterol Hepatol (N Y). 2015;11(8):554-556.
- Loddo I, Romano C. Inflammatory Bowel Disease: Genetics, Epigenetics, and Pathogenesis. Front Immunol. 2015;6:551.
doi pubmed - Uhlig HH. Monogenic diseases associated with intestinal inflammation: implications for the understanding of inflammatory bowel disease. Gut. 2013;62(12):1795-1805.
doi pubmed - Bianco AM, Girardelli M, Tommasini A. Genetics of inflammatory bowel disease from multifactorial to monogenic forms. World J Gastroenterol. 2015;21(43):12296-12310.
doi pubmed - Hutchins AP, Diez D, Miranda-Saavedra D. The IL-10/STAT3-mediated anti-inflammatory response: recent developments and future challenge. Brif Funct Genomics. 2013;12:489-498.
doi pubmed - Ding Y, Qin L, Zamarin D, Kotenko SV, Pestka S, Moore KW, Bromberg JS. Differential IL-10R1 expression plays a critical role in IL-10-mediated immune regulation. J Immunol. 2001;167(12):6884-6892.
doi pubmed - Commins S, Steinke JW, Borish L. The extended IL-10 superfamily: IL-10, IL-19, IL-20, IL-22, IL-24, IL-26, IL-28, and IL-29. J Allergy Clin Immunol. 2008;121(5):1108-1111.
doi pubmed - Murray PJ. The primary mechanism of the IL-10-regulated antiinflammatory response is to selectively inhibit transcription. Proc Natl Acad Sci U S A. 2005;102(24):8686-8691.
doi pubmed - Engelhardt KR, Grimbacher B. IL-10 in humans: lessons from the gut, IL-10/IL-10 receptor deficiencies, and IL-10 polymorphisms. Curr Top Microbiol Immunol. 2014;380:1-18.
doi pubmed - Glocker EO, Kotlarz D, Boztug K, Gertz EM, Schaffer AA, Noyan F, Perro M, et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med. 2009;361(21):2033-2045.
doi pubmed - Begue B, Verdier J, Rieux-Laucat F, Goulet O, Morali A, Canioni D, Hugot JP, et al. Defective IL10 signaling defining a subgroup of patients with inflammatory bowel disease. Am J Gastroenterol. 2011;106(8):1544-1555.
doi pubmed - Mao H, Yang W, Lee PP, Ho MH, Yang J, Zeng S, Chong CY, et al. Exome sequencing identifies novel compound heterozygous mutations of IL-10 receptor 1 in neonatal-onset Crohn's disease. Genes Immun. 2012;13(5):437-442.
doi pubmed - Kotlarz D, Beier R, Murugan D, Diestelhorst J, Jensen O, Boztug K, Pfeifer D, et al. Loss of interleukin-10 signaling and infantile inflammatory bowel disease: implications for diagnosis and therapy. Gastroenterology. 2012;143(2):347-355.
doi pubmed - Shim JO, Hwang S, Yang HR, Moon JS, Chang JY, Ko JS, Park SS, et al. Interleukin-10 receptor mutations in children with neonatal-onset Crohn's disease and intractable ulcerating enterocolitis. Eur J Gastroenterol Hepatol. 2013;25(10):1235-1240.
doi - Moran CJ, Walters TD, Guo CH, Kugathasan S, Klein C, Turner D, Wolters VM, et al. IL-10R polymorphisms are associated with very-early-onset ulcerative colitis. Inflamm Bowel Dis. 2013;19(1):115-123.
doi pubmed - Neven B, Mamessier E, Bruneau J, Kaltenbach S, Kotlarz D, Suarez F, Masliah-Planchon J, et al. A Mendelian predisposition to B-cell lymphoma caused by IL-10R deficiency. Blood. 2013;122(23):3713-3722.
doi pubmed - Pigneur B, Escher J, Elawad M, Lima R, Buderus S, Kierkus J, Guariso G, et al. Phenotypic characterization of very early-onset IBD due to mutations in the IL10, IL10 receptor alpha or beta gene: a survey of the Genius Working Group. Inflamm Bowel Dis. 2013;19(13):2820-2828.
doi pubmed - Galatola M, Miele E, Strisciuglio C, Paparo L, Rega D, Delrio P, Duraturo F, et al. Synergistic effect of interleukin-10-receptor variants in a case of early-onset ulcerative colitis. World J Gastroenterol. 2013;19(46):8659-8670.
doi pubmed - Murugan D, Albert MH, Langemeier J, Bohne J, Puchalka J, Jarvinen PM, Hauck F, et al. Very early onset inflammatory bowel disease associated with aberrant trafficking of IL-10R1 and cure by T cell replete haploidentical bone marrow transplantation. J Clin Immunol. 2014;34(3):331-339.
doi pubmed - Shim JO, Seo JK. Very early-onset inflammatory bowel disease (IBD) in infancy is a different disease entity from adult-onset IBD; one form of interleukin-10 receptor mutations. J Hum Genet. 2014;59(6):337-341.
doi pubmed - Lee CH, Hsu P, Nanan B, Nanan R, Wong M, Gaskin KJ, Leong RW, et al. Novel de novo mutations of the interleukin-10 receptor gene lead to infantile onset inflammatory bowel disease. J Crohns Colitis. 2014;8(11):1551-1556.
doi pubmed - Beser OF, Conde CD, Serwas NK, Cokugras FC, Kutlu T, Boztug K, Erkan T. Clinical features of interleukin 10 receptor gene mutations in children with very early-onset inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2015;60(3):332-338.
doi pubmed - Lu D, Xu Y, Chen Y, Zeng P, Chen H, Zeng H. [Interleukin-10 receptor mutations in children with neonatal onset inflammatory bowel disease: genetic diagnosis and pathogenesis]. Zhonghua Er Ke Za Zhi. 2015;53(5):348-354.
pubmed - Kelsen JR, Dawany N, Moran CJ, Petersen BS, Sarmady M, Sasson A, Pauly-Hubbard H, et al. Exome sequencing analysis reveals variants in primary immunodeficiency genes in patients with very early onset inflammatory bowel disease. Gastroenterology. 2015;149(6):1415-1424.
doi pubmed - Yanagi T, Mizuochi T, Takaki Y, Eda K, Mitsuyama K, Ishimura M, Takada H, et al. Novel exonic mutation inducing aberrant splicing in the IL10RA gene and resulting in infantile-onset inflammatory bowel disease: a case report. BMC Gastroenterol. 2016;16:10.
doi pubmed - Oh SH, Baek J, Liany H, Foo JN, Kim KM, Yang SC, Liu J, et al. A Synonymous Variant in IL10RA Affects RNA Splicing in Paediatric Patients with Refractory Inflammatory Bowel Disease. J Crohns Colitis. 2016;10(11):1366-1371.
doi pubmed - Xiao Y, Wang XQ, Yu Y, Guo Y, Xu X, Gong L, Zhou T, et al. Comprehensive mutation screening for 10 genes in Chinese patients suffering very early onset inflammatory bowel disease. World J Gastroenterol. 2016;22(24):5578-5588.
doi pubmed - Kammermeier J, Dziubak R, Pescarin M, Drury S, Godwin H, Reeve K, Chadokufa S, et al. Phenotypic and Genotypic Characterisation of Inflammatory Bowel Disease Presenting Before the Age of 2 years. J Crohns Colitis. 2017;11(1):60-69.
doi pubmed - Engelhardt KR, Shah N, Faizura-Yeop I, Kocacik Uygun DF, Frede N, Muise AM, Shteyer E, et al. Clinical outcome in IL-10- and IL-10 receptor-deficient patients with or without hematopoietic stem cell transplantation. J Allergy Clin Immunol. 2013;131(3):825-830.
doi pubmed - Gassas A, Courtney S, Armstrong C, Kapllani E, Muise AM, Schechter T. Unrelated donor hematopoietic stem cell transplantation for infantile enteropathy due to IL-10/IL-10 receptor defect. Pediatr Transplant. 2015;19(4):E101-103.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


