| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website http://www.gastrores.org |
Original Article
Volume 3, Number 3, June 2010, pages 106-111
Effects of Gelatinization of Enteral Nutrients on Human Gastric Emptying
Naruo Kawasakia, b, d, Mitsuyoshi Urashimac, Hironori Odairaa, Takuji Noroa, Yutaka Suzukia
aDepartment of Surgery, International University of Health and Welfare Hospital, Japan
bDepartment of Surgery, Jikei University School of Medicine, Japan
cDivision of Clinical Research and Development, Jikei University School of Medicine, Japan
dCorresponding author: 537-3, Iguchi, Nasusiobara, Tochigi 329-2763, Department of Surgery, International University of Health and Welfare, Japan
Manuscript accepted for publication June 16, 2010
Short title: Gelatinization and Gastric Emptying
doi: https://doi.org/10.4021/gr2010.06.213w
| Abstract | ▴Top |
Background: Gastrointestinal side effects, particularly diarrhea, are still the main reasons for discontinuation of enteral nutrition. Gelatinization of liquid meal for the prevention of diarrhea has been reported as effective. The purpose of this study was to investigate the effects of gelatinization of liquid meal on gastric emptying.
Methods: Ten healthy volunteers were studied two times, with 2-week interval between tests. The total calorific value was set at 225 kcal, and 3 test meals were prepared: liquid meal and 2 types of gelatinized meals. These 2 types of gelatinized meals are different viscosity. 13C-sodium acetate (100 mg) was thoroughly mixed, and exhaled air was sampled. The results of gastric emptying were expressed as the time of peak excretion (Tmax), and absorption was expressed as the area under the 13CO2 curve up to Tmax (AUC-Tmax). At the same time, blood samples were collected to measure levels of blood glucose, insulin and gastrin.
Results: The mean value of Tmax were 52.0, 77.3 and 85.6 min. Compared to liquid meal, gastric emptying for gelatinized meals was significantly delayed. The mean value of AUC-Tmax were 22.7, 28.7 and 33.7%dose, respectively, and no significant differences in absorption were seen. No significant differences existed in blood glucose, gastrin and insulin.
Conclusions: Gelatinization of liquid meal delays gastric emptying. Gelatinized liquid meal may be useful for the management of diarrhea accompanied with enteral nutrition without influencing gastrointestinal hormone and blood glucose.
Keywords: Gelatinization; Gastric emptying; Absorption; 13C breath test; Enteral nutrition
| Introduction | ▴Top |
The patients, who cannot orally consume the necessary amount of nutrients, are expanding. In supplementary nutrition, nutrients are administered from a nasogastric tube or gastrostomy, but clinically relevant complications such as gastroesophageal reflux, aspiration pneumonia and diarrhea are likely to occur [1, 2]. Gastrointestinal side effects, particularly diarrhea, are still the main reasons for discontinuation of enteral nutrition. The composition and osmotic pressure of enteral nutrition solution is frequently suspected of playing a leading role in causing diarrhea [3]. The management of diarrhea requires knowledge of gastro-intestinal physiology during enteral nutrition.
While gelatinization of liquid meal for the prevention of these complications has been reported as effective [4], no systematic assessments or scientific evidence support the efficacy of this approach. We used the 13C breath test to ascertain the effects of liquid meal and gelatinized meals on gastric emptying, absorption and gastrointestinal hormones.
| Materials and Methods | ▴Top |
Subjects and test meals
Subjects comprised 10 healthy volunteers [3 men, 7 women; age 28.6 ± 6.7 years; BMI, 20.6 ± 1.7 (mean ± SD)] who underwent the 13C breath test after 12 hours of fasting with ≥ 1 week of break between each test. Enteral nutrients were prepared using liquid meal (semi-digested nutritional supplement, 1 kcal/ml; Racol, Otsuka pharmaceutical, Tokyo, Japan) and Easygel gelatinizing agent, consisting of pectin and calcium lactate (total calorific value: 25 kcal; Otsuka pharmaceutical, Tokyo, Japan). Informed consent was obtained from all subjects prior to enrollment in this study.
The two nutritional formulas were well balanced for nitrogen and electrolyte. The total calorific value of each test meal was set at 225 kcal. Table 1 shows the composition of each test meal: (a) Liquid meal: 225 ml; (b) Gelatinized meal 1:200 ml of liquid meal and 25 kcal of gelatinizing agent (viscosity: 16,000 ± 800 cp); (c) Gelatinized meal 2:175 ml of liquid meal and 50 kcal of gelatinizing agent (viscosity: 128,000 ± 4,300 cp).
 Click to view | Table 1. Composition of Test Meals |
With each test meal, 100 mg of 13C-sodium acetate (13C-labeled compound) was thoroughly mixed. Viscosity of the test meal was measured using a Type B rotating viscometer (VDA; Shibaura System, Tokyo, Japan) after stirring for 2 min at 12 rpm. Measurements were taken in triplicate at 20 ± 2 °C.
Test meals were consumed within 5 min of preparation, and 20 ml of water was consumed to flush down any residual meal in the esophagus.
Measurement methods
Expired air was sampled before and 5, 10, 15, 20, 30, 40, 50, 60, 75, 90, 105, 120, 135, 150, 165, 180, 210 and 240 min after intake. The labeled meal was emptied from the stomach, absorbed through the duodenum, metabolized in the liver and then exhaled. The amount of 13CO2 in expired air was measured using an UBiT-IR300 (Fukuda Denshi, Tokyo, Japan).
Assessment indicators
Gastric emptying
According to the standard gastric emptying procedure in the 13C breath test as published by the Japan Society of Smooth Muscle Research, Tmax (Time of the highest %dose on the 13CO2 curve) was used to assess gastric emptying.
Absorption
Area under the 13CO2 curve up to Tmax (AUC-Tmax: %dose) was used to assess absorption.
Blood biochemistry
Blood glucose, insulin and gastrin were measured by collecting a blood sample before and 30, 60, 90, 120, 150 and 180 min after intake.
Statistical analysis
The results were expressed as mean ± standard deviation (SD). The effects of intervention compared with control (liquid meal) were computed by repeated measure analysis of variance using STATA 8.0. Statistical significance was set at P < 0.05 (Stat View 5.0; SAS Institute Inc, USA).
| Results | ▴Top |
Gastric emptying
Mean durations of gastric emptying for liquid meal, gelatinized meal 1 and 2 were 52.0 ± 10.1, 77.3 ± 20.8 and 85.6 ± 25.0 min, respectively. Compared to liquid meal, gastric emptying for gelatinized meals was significantly delayed (P < 0.05; Fig. 1).
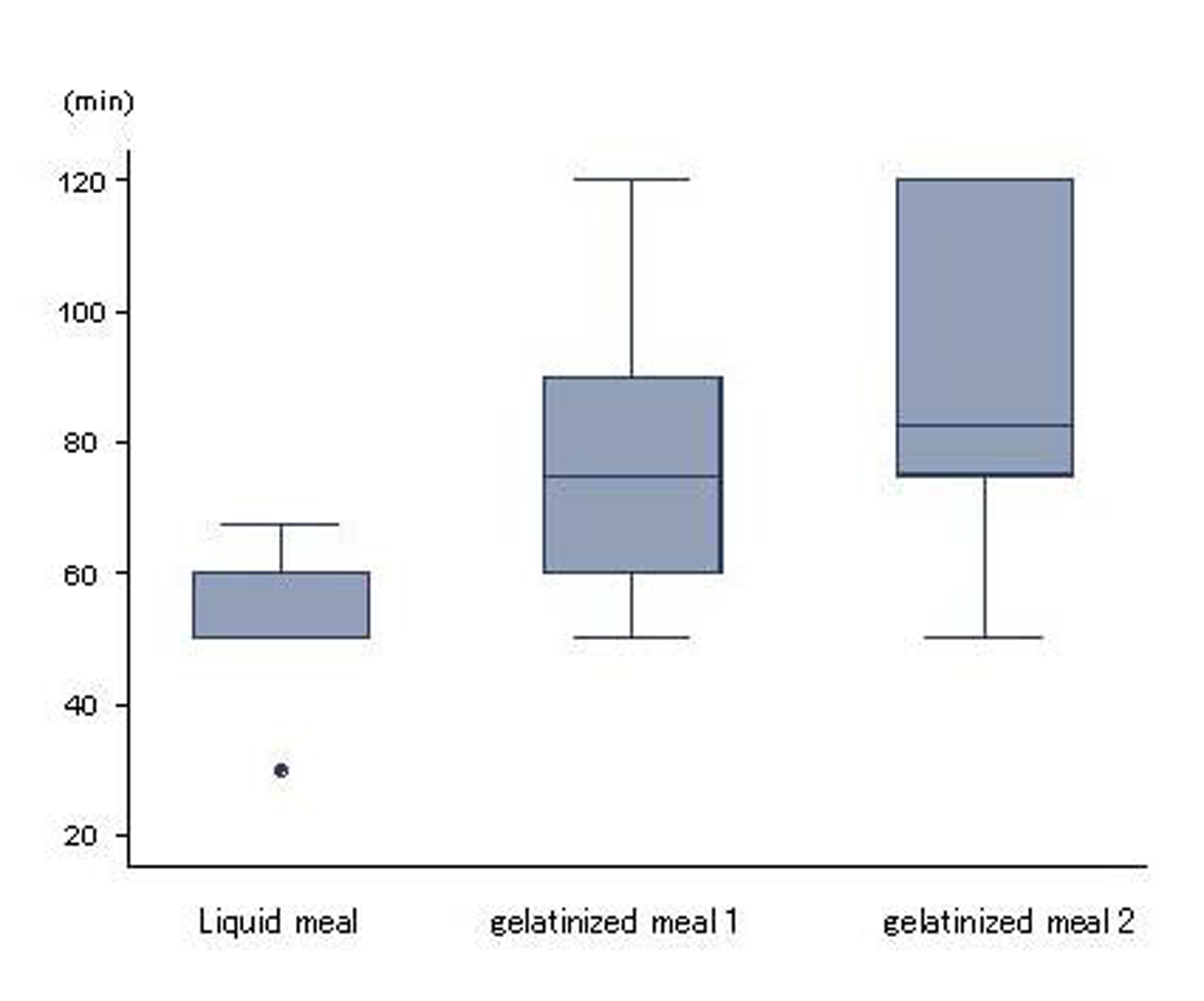 Click for large image | Figure 1. Gastric emptying. Liquid and gelatinized meals displayed differences in gastric emptying (p < 0.05). Higher viscosity was associated with slower gastric emptying. Results are expressed as mean ± SD. |
Absorption
The %dose for liquid meal, gelatinized meal 1 and 2 were 22.7 ± 6.1%, 28.7 ± 8.7% and 33.7 ± 12.7%, respectively, and no significant differences in absorption were seen between liquid and gelatinized meals (Fig. 2).
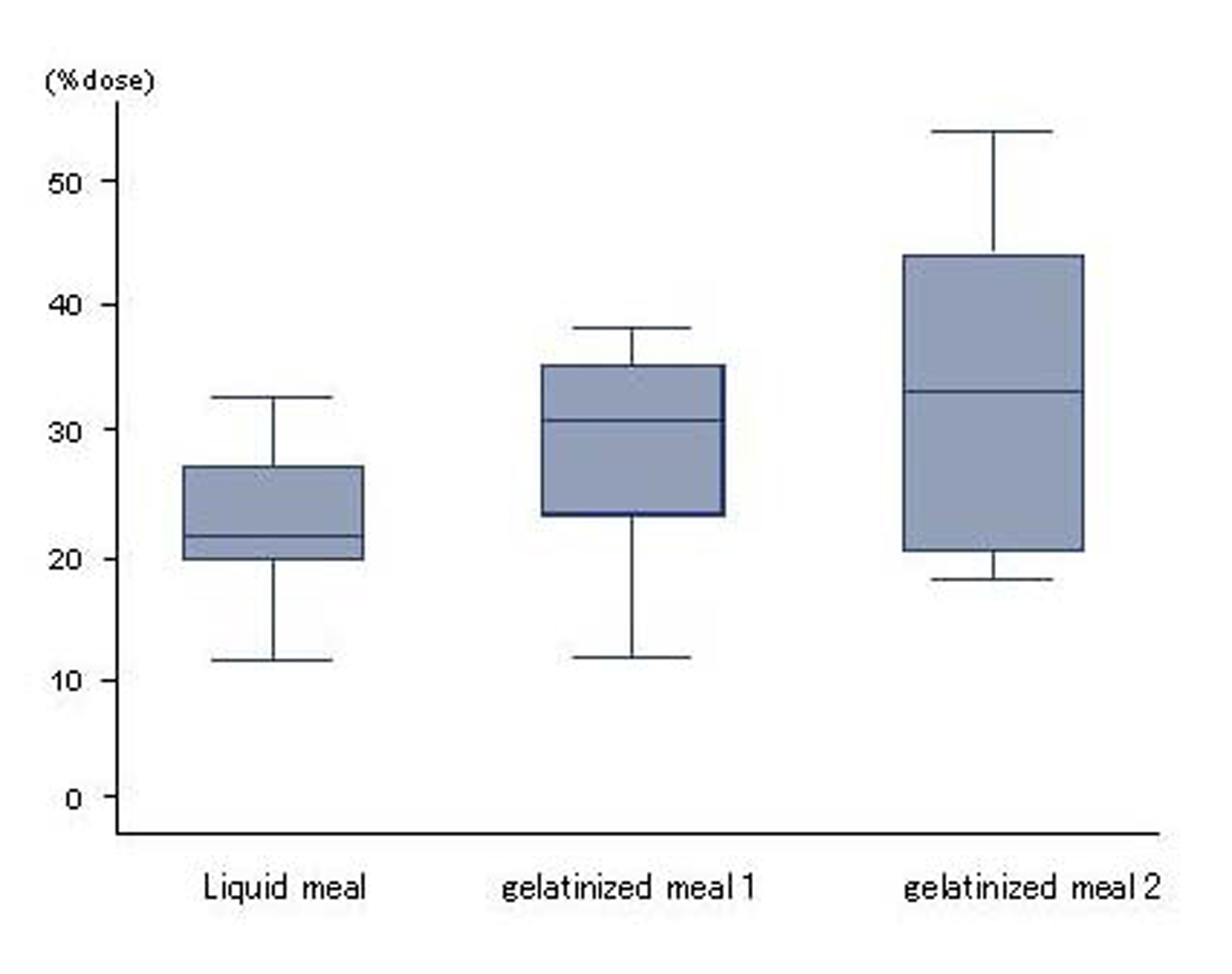 Click for large image | Figure 2. Absorption. No differences in absorption were seen between liquid and gelatinized meals. Results are expressed as mean ± SD. |
Blood biochemistry
With all test meals, blood glucose and gastrin peaked 30 min after intake and insulin peaked 60 min after intake, then decreased over time. No significant differences among test meals were seen (Fig. 3).
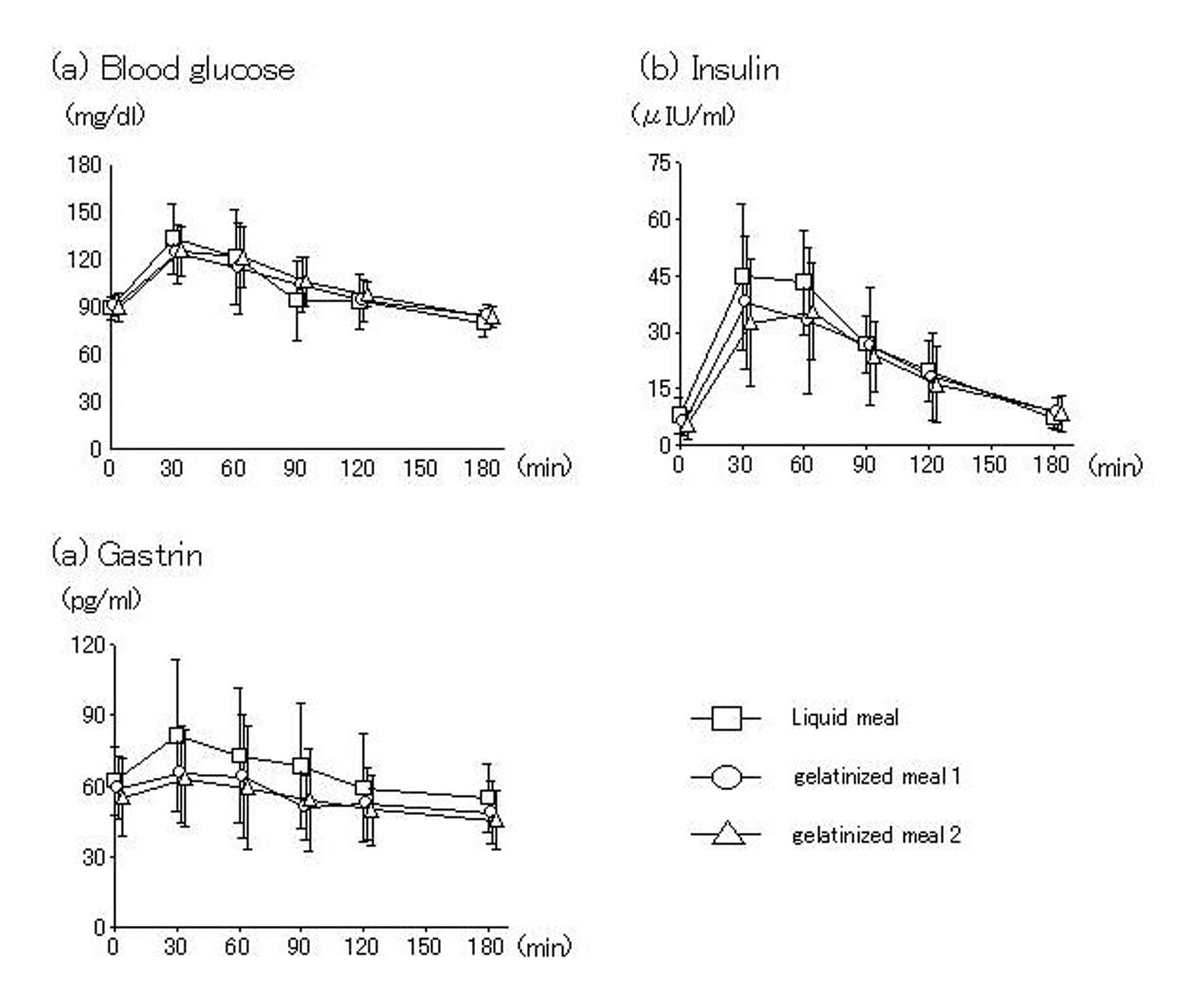 Click for large image | Figure 3. Blood glucose and gastrointestinal hormone. No differences in blood levels of glucose, insulin or gastrin were seen between liquid and gelatinized meals. |
| Discussion | ▴Top |
The gelatinization of enteral nutrients is believed to be useful for preventing complications such as gastroesophageal reflux, aspiration pneumonia and diarrhea [1]. However, most past studies were empirical, and scientific evidence to support the efficacy of this approach has been lacking.
Gastric emptying can be measured directly by imaging modalities such as the radioisotope method and the opaque marker method, or indirectly by mixing a test meal and a reagent, such as acetaminophen, sulfamethizole or 13C, and allowing the mixture to be absorbed through the small intestine.
The 13C method is a gastric emptying test using a compound labeled with 13C, a non-radioactive stable isotope. Ever since the first report by Ghoos et al [5], this method has been performed widely, although mostly in Western countries [6, 7]. Also, the 13C method correlates closely with the radioisotope method, which is considered the golden standard for gastric emptying measurement, and is convenient and involves no radiation exposure [8, 9]. In Japan, studies have been conducted based on the standard guidelines published by academic societies such as the Japan Society of Smooth Muscle Research [10]. As a test meal, a liquid meal (200 kcal, 1 kcal/ml) thoroughly mixed with 10 mm of 13C-sodium acetate (13C-labeled compound) is recommended, and the present study followed this guideline.
When assessing gastric emptying, the duration for half of a test meal to empty (T1/2) [5] and the duration for 13CO2 in expired air to peak (Tmax) [6] have been used. Along a 13CO2 curve, duration to reach Tmax is mostly related to gastric emptying and absorption of a 13C-labeled compound, while the time after Tmax represents metabolism and excretion. In many cases, T1/2 is after Tmax, and when compared to T1/2, Tmax is thought to more closely reflect gastric emptying due to the effects of the metabolism and excretion of a 13C-labeled compound.
In digestion and absorption tests using 13C-labeled compounds, long-chain fatty acids such as triolein, palmitic acid and trioctanoin, and their ester substrates, have been utilized [11-13], but digestion and absorption cannot be simultaneously assessed with gastric emptying. Most nutrients are absorbed through the small intestine, and in the stomach, nutrients are digested and then emptied. Gastric emptying and absorption should thus affect each other, and simultaneous assessment enables evaluation of gastrointestinal function. In the past, the concentration curve of a labeled-compound was used to calculate area under the entire curve and peak values of the labeled compound. However, the area under the entire curve also reflects the metabolism and excretion of a 13C-labeled compound. In addition, following upper gastrointestinal tract resection such as distal gastrectomy and increased gastric emptying can increase apparent absorption. Establishing indicators that are less likely to be affected by gastric emptying is thus necessary. We mentioned above that, from 13CO2 curves, the duration to reach Tmax can be seen as the amount of time required to empty and absorb a 13C-labeled compound. Based on this notion, we assess food absorption based on the area under the curve up to Tmax.
Based on the present results, the gelatinization of liquid meal delays gastric emptying, but does not affect absorption or gastrointestinal hormone levels.
Gastric emptying is also markedly affected by calories [14], but is less affected by the composition and osmotic pressure of food [15]. In terms of food characteristics, liquid meals are emptied faster than solid meals [16], because of decreased relaxation of the proximal stomach [17, 18]. Gelatinization delays gastric emptying by transiently increasing the particle size of liquid meal, thus making breakdown of food more difficult. Gastric emptying of gelatinized liquid meal resembles that of solid meal.
Increased gastric emptying is thought to be related to diarrhea [19], and favorable absorption requires nutrients to come in contact with the small intestinal mucosa. While gelatinization does not affect absorption, gradual gastric emptying makes nutrients pass through the small intestine slower, thus preventing diarrhea.
Acknowledgments
This study was sponsored by Otsuka pharmaceutical Company.
Conflict of Interest
Each of authors has no conflicts of interest about the contents in this study.
| References | ▴Top |
- Galindo-Ciocon DJ. Tube feeding: complications among the elderly. J Gerontol Nurs. 1993;19(6):17-22.
pubmed - Ciocon JO, Galindo-Ciocon DJ, Tiessen C, Galindo D. Continuous compared with intermittent tube feeding in the elderly. JPEN J Parenter Enteral Nutr. 1992;16(6):525-528.
pubmed doi - Homann HH, Kemen M, Fuessenich C, Senkal M, Zumtobel V. Reduction in diarrhea incidence by soluble fiber in patients receiving total or supplemental enteral nutrition. JPEN J Parenter Enteral Nutr. 1994;18(6):486-490.
pubmed doi - Kanie J, Suzuki Y, Iguchi A, Akatsu H, Yamamoto T, Shimokata H. Prevention of gastroesophageal reflux using an application of half-solid nutrients in patients with percutaneous endoscopic gastrostomy feeding. J Am Geriatr Soc. 2004;52(3):466-467.
pubmed doi - Ghoos YF, Maes BD, Geypens BJ, Mys G, Hiele MI, Rutgeerts PJ, Vantrappen G. Measurement of gastric emptying rate of solids by means of a carbon-labeled octanoic acid breath test. Gastroenterology. 1993;104(6):1640-1647.
pubmed - Braden B, Adams S, Duan LP, Orth KH, Maul FD, Lembcke B, Hor G,
et al . The [13C]acetate breath test accurately reflects gastric emptying of liquids in both liquid and semisolid test meals. Gastroenterology. 1995;108(4):1048-1055.
pubmed doi - Choi MG, Camilleri M, Burton DD, Zinsmeister AR, Forstrom LA, Nair KS. [13C]octanoic acid breath test for gastric emptying of solids: accuracy, reproducibility, and comparison with scintigraphy. Gastroenterology. 1997;112(4):1155-1162.
pubmed doi - Yasuhito Sasaki. The breath test by CO2 analysis. Progress of breath tests using isotopes of carbon in Japan. Radioisotopes. 1991;40:475-84.
- Makiko Suehiro, Norio Sakuragawa, Hiroshi Nishida. The breath test by CO2 analysis. Basic methodology of 13C breath test. Radioisotopes. 1991;40:535-44.
- Nakada K, Aoyama N, Nakagawa M, Kawasaki N, Shirasaka D, Zai H, Urita S,
et al . The report of the workshop entitled “The present and the future in gestric emptying study assessed by 13C breath test with special referrence to the standardization of the method” at the 44th annual meeting of the Japanese Smooth Muscle Society. J Smooth Musclr Res(Jpn. Sec.). 2002;6:J75-J91. - Watkins JB, Klein PD, Schoeller DA, Kirschner BS, Park R, Perman JA. Diagnosis and differentiation of fat malabsorption in children using 13C-labeled lipids: trioctanoin, triolein, and palmitic acid breath tests. Gastroenterology. 1982;82(5 Pt 1):911-917.
pubmed - Vantrappen GR, Rutgeerts PJ, Ghoos YF, Hiele MI. Mixed triglyceride breath test: a noninvasive test of pancreatic lipase activity in the duodenum. Gastroenterology. 1989;96(4):1126-1134.
pubmed - Hinder RA, Kelly KA. Canine gastric emptying of solids and liquids. Am J Physiol. 1977;233(4):E335-340.
pubmed - McHugh PR, Moran TH. Calories and gastric emptying: a regulatory capacity with implications for feeding. Am J Physiol. 1979;236(5):R254-260.
pubmed - Kalogeris TJ, Reidelberger RD, Mendel VE. Effect of nutrient density and composition of liquid meals on gastric emptying in feeding rats. Am J Physiol. 1983;244(6):R865-871.
pubmed - Kawasaki N, Nakada K, Hanyu N, Yanai S, Takahashi T, Nakao M. Comparison of gastric emptying among different kind of food by RI method. Comparison of gastric emptying between liquid and solid component of the same test meal by performing RI and 13C method simultaneously. Comparison of gastric emptying of the liquid meal among RI, 13C and APAP method. J Smooth Musclr Res(Jpn. Sec.). 2002;6:J99-J106.
- Jahnberg T, Martinson J, Hulten L, Fasth S. Dynamic gastric response to expansion before and after vagotomy. Scand J Gastroenterol. 1975;10(6):593-598.
pubmed - Stadaas JO. Intragastric pressure/volume relationship before and after proximal gastric vagotomy. Scand J Gastroenterol. 1975;10(2):129-134.
pubmed - Parr NJ, Grime S, Brownless S, Critchley M, Baxter JN, Mackie CR. Relationship between gastric emptying of liquid and postvagotomy diarrhoea. Br J Surg. 1988;75(3):279-282.
pubmed doi
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


