| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Case Report
Volume 16, Number 3, June 2023, pages 184-191
Does Liver Resection Remain a Viable Option in Patients With Pyogenic Liver Abscess? A Single-Center Experience
Aiman Obeda, Mohammad Abuassib, f, Saqr Alsakarnehc, f, Fouad Jaberc, g, Mahmoud Fakhrid, Fadi Abufaresd, Abdalla Bashird, Mahmood Syamd, Anwar Jarrada, Ody Abdelhadid, Hassan Ghoze
aHepatobiliary and Transplant Surgery Department, Jordan Hospital, Amman, Jordan
bInternal Medicine Department, Jordan Hospital, Amman, Jordan
cInternal Medicine Department, University of Missouri-Kansas City, Kansas City, MO, USA
dGeneral Surgery Department, Jordan Hospital, Amman, Jordan
eGastroenterology Department, University of Missouri-Kansas City, Kansas City, MO, USA
fThese authors contributed equally to this study.
gCorresponding Author: Fouad Jaber, Internal Medicine Department, University of Missouri-Kansas City, Kansas City, MO 64108, USA
Manuscript submitted March 21, 2023, accepted May 2, 2023, published online June 11, 2023
Short title: Liver Resection for Pyogenic Liver Abscess
doi: https://doi.org/10.14740/gr1611
| Abstract | ▴Top |
Pyogenic liver abscesses (PLAs) are relatively rare but often fatal if left untreated. Antibiotic therapy combined with percutaneous procedures has replaced surgery as the cornerstone of treatment. However, open surgical drainage or liver resection may be a last resort. This study aimed to review our experience in treating PLA, with a focus on the conditions requiring partial liver resection as the last viable curative option. Medical records of patients with PLA admitted to Jordan Hospital between October 2014 through October 2020 were retrospectively reviewed. Medical and demographic data of all 43 patients admitted to our facility with a diagnosis of PLA were extracted. We reviewed these patients and extracted the cases that required surgical intervention. Four (three males and one female) of the 43 patients with PLA required surgical intervention. The underlying causes of liver abscesses were as follows: one traumatic due to shrapnel injury from an explosion, one following chemoembolization for hepatocellular carcinoma, and two patients with no apparent etiology. All patients were diagnosed with a computed tomography (CT) scan of the abdomen and pelvis with intravenous contrast. Two patients had negative cultures. All patients received broad-spectrum antibiotics, and all underwent CT- or ultrasound-guided percutaneous drainage or aspiration. All four patients required partial hepatic resection due to treatment failure or inaccessible percutaneous procedures with clinical improvement. Although antimicrobial and interventional therapy remains the primary treatment option in PLA, the surgical option with open surgical drainage or partial liver resection remains viable and curative in selected patients.
Keywords: Liver abscess; Liver resection; Interventional therapy; Antibiotics
| Introduction | ▴Top |
Pyogenic liver abscess (PLA) is a life-threatening disease with a mortality rate of 11-31% and almost 10,000 acute morbidities per year [1]. PLA is accompanied by risk factors and chronic diseases such as cardiovascular diseases, malignancies, diabetes mellitus, liver cirrhosis, cholangitis, urinary tract diseases, pneumonia, autoimmune diseases, and malnutrition [2]. However, up to 55% of patients with PLA have no known risk factors [3]. Clinical manifestations are initially nonspecific and easily misdiagnosed.
Diagnosing and treating liver abscesses can be challenging. Antibiotic therapy is usually carried out first, followed by ultrasound (US) or computed tomography (CT)-guided percutaneous drainage or aspiration [4]. Historically, surgical drainage was the only adjunct to antimicrobial therapy. Systemic antibiotics and percutaneous intervention are efficient and safe for curing most liver abscesses [5]. Nevertheless, a minority of liver abscesses may require surgical intervention, mainly partial liver resection [5]. In these situations, the surgical option provides a final life-saving anchor with source elimination.
This study retrospectively reviewed our 5-year experience of 43 PLA patients diagnosed and treated at Jordan Hospital. We present the four cases that required surgical intervention to uncover the risk factors, common pathogens, and the surgical option as the last viable curative option.
| Case Reports | ▴Top |
Methods
Our institution’s ethics committee granted ethical approval to this study, which was then conducted per the Declaration of Helsinki. Demographic and clinical data of all patients admitted to our hospital with liver abscesses between October 2014 through October 2020 were retrospectively entered into a database. The data were obtained from a combination of chart reviews and records at the Jordan Hospital. These data were reviewed to document clinical presentation, etiology, diagnostic workup, treatment, morbidity, and mortality. Aspiration material at the time of primary abscess drainage was regularly sent for microbial culture and sensitivity analysis.
Case 1
A 75-year-old male with a past medical history of atrial fibrillation, chronic hepatitis B, liver cirrhosis, and hepatocellular carcinoma (HCC) status post-trans-arterial chemoembolization (TACE) 3 months prior presented with upper abdominal pain, abdominal distension, and anorexia. The physical exam was remarkable for a febrile patient with scleral icterus. The abdomen was distended with tenderness to palpation in the right upper quadrant (RUQ) without rebound tenderness or rigidity and with positive bowel sounds. Laboratories were remarkable for a leukocytosis of 17.6 × 103 cells/dL, C-reactive protein (CRP) of 249, albumin of 3.1, alkaline phosphatase (ALP) of 70, and international normalized ratio (INR) of 2. Alanine transaminase (ALT) and aspartate transaminase (AST) were normal, and blood cultures were negative. He was initially treated conservatively via nasogastric tube insertion, intravenous (IV) hydration, and IV antibiotics. Abdominal and pelvic CT scan with IV contrast showed a fluid density of 7 × 5 cm with gas pockets in segment VII peripherally associated with moderate ascites and multiple gas pockets in the right subdiaphragmatic region (Fig. 1). CT-guided aspiration of the abscess was performed, and a small amount of thick gelatinous bloody fluid was aspirated and sent for culture and sensitivity (Fig. 2). Aspiration drainage grew Enterococcus faecium. The patient’s clinical condition deteriorated, and he developed multiple peaks of high-grade fever, atrial fibrillation with a rapid ventricular response, hypotension, and altered mental status. CT-guided drainage of the liver abscess was challenging since the abscess was large, and the decision to surgically drain the abscess was pursued. The patient underwent surgery through an inverted L-abdominal incision and was found to have a large subphrenic abscess extending from the anterolateral surface of the liver posteriorly to segment VII, contiguous with the intrahepatic abscess (segment VII). Both abscesses were drained along with the resection of segment VII. The cultures grew Escherichia coli (extended spectrum beta-lactamase). His clinical condition remains stable postoperatively, and he was discharged on the sixth postoperative day. Follow-up CRP was 39, and CT of the abdomen and pelvis 3 months later showed enlarged liver with stigmata of cirrhosis with no clear evidence of a focal liver lesion (Fig. 3).
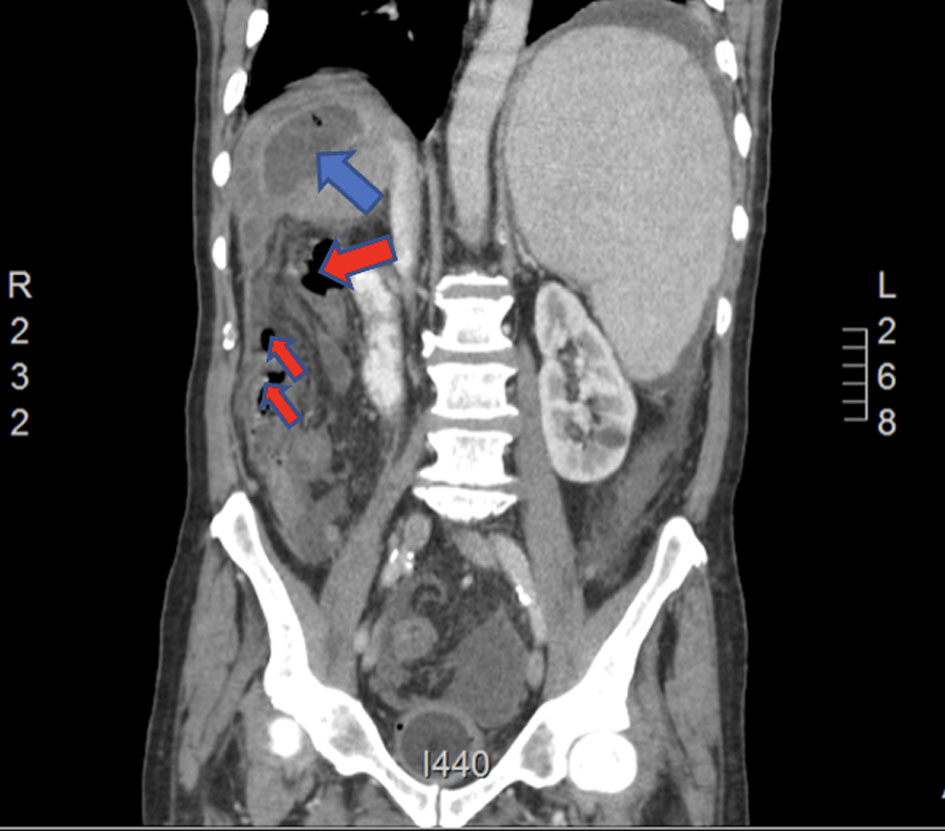 Click for large image | Figure 1. Coronal CT of abdomen and pelvis on presentation with IV contrast showing a 7 × 5 cm fluid density with gas pockets in segment VII peripherally (blue arrow) associated with moderate ascites and multiple gas pockets in the right subhepatic region (red arrows). CT: computed tomography. |
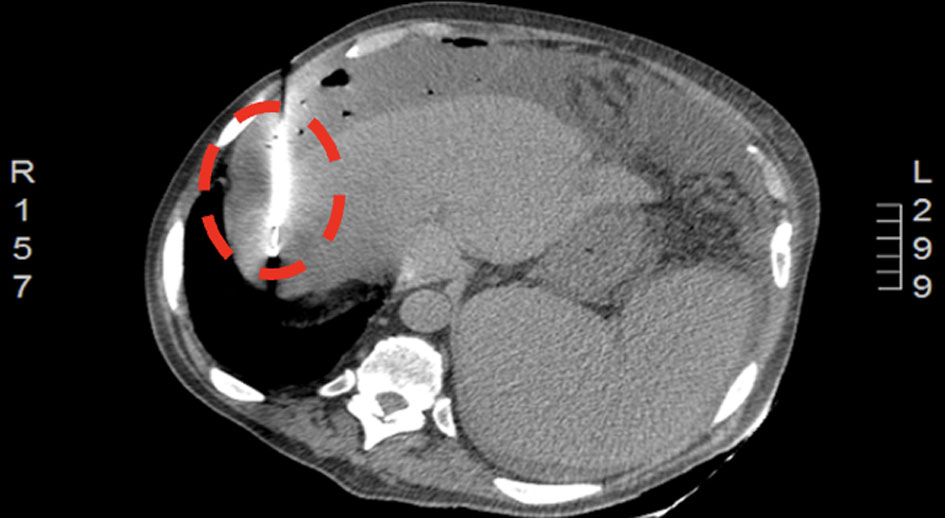 Click for large image | Figure 2. CT of abdomen 9 days after abscess drainage showing a percutaneous drain in segment VII (encircled). CT: computed tomography. |
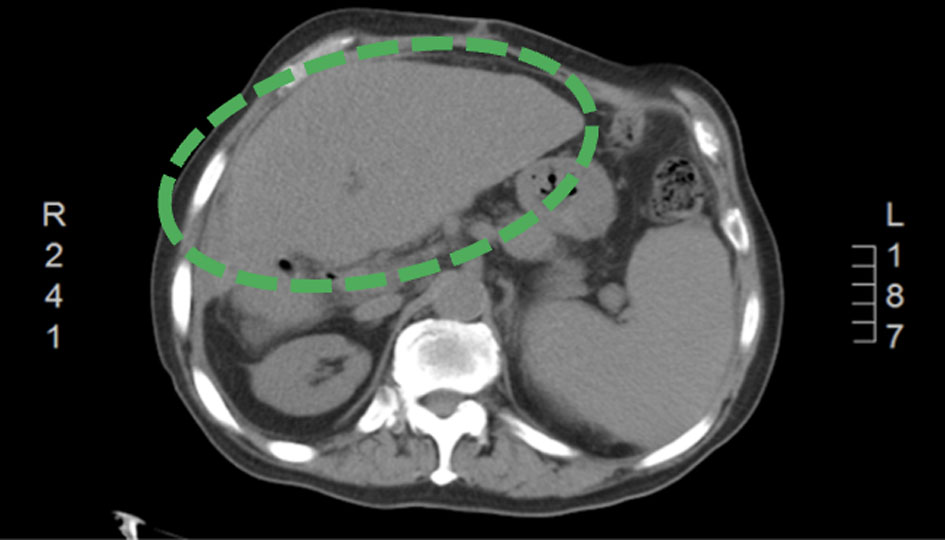 Click for large image | Figure 3. A follow-up CT of abdomen and pelvis (3 months after discharge) showing enlarged cirrhotic liver without definite evidence of a focal liver lesion (green encircled). CT: computed tomography. |
Case 2
A 48-year-old female with a past medical history of nonspecific inflammatory cecal mass and recurrent multiple liver abscesses presented with epigastric pain, abdominal distention, and fever. CT of abdomen and pelvis with IV contrast 2 months prior showed numerous cystic lesions scattered in both lobes of the liver, with the largest measuring 12 × 10-compressing segment VII (Figs. 4, 5). She failed 6 weeks of IV antibiotics and two attempts at percutaneous liver abscess drainage (Figs. 4, 5). Physical examination was notable for a soft abdomen and tenderness in the RUQ area, with no rebound tenderness or rigidity. Laboratory evaluation was notable for leukocytosis of 14.4 × 103 cells/dL (with a shift to the lift), a CRP of 240, albumin of 2.8, and ALP of 46. Blood cultures were negative. CT of abdomen and pelvis showed a recurrence of abscess in segment VII measuring 11.6 × 10.4 cm (Fig. 6). CT-guided drainage of the right liver abscess was performed with negative aspirated fluid cultures. Due to treatment failure, the surgical option was planned. The patient underwent a cholecystectomy with resection of segment VII and most of segment VI through the right abdominal subcostal incision. Follow-up ultrasound 3 weeks later showed complete resolution with no evidence of liver abscess.
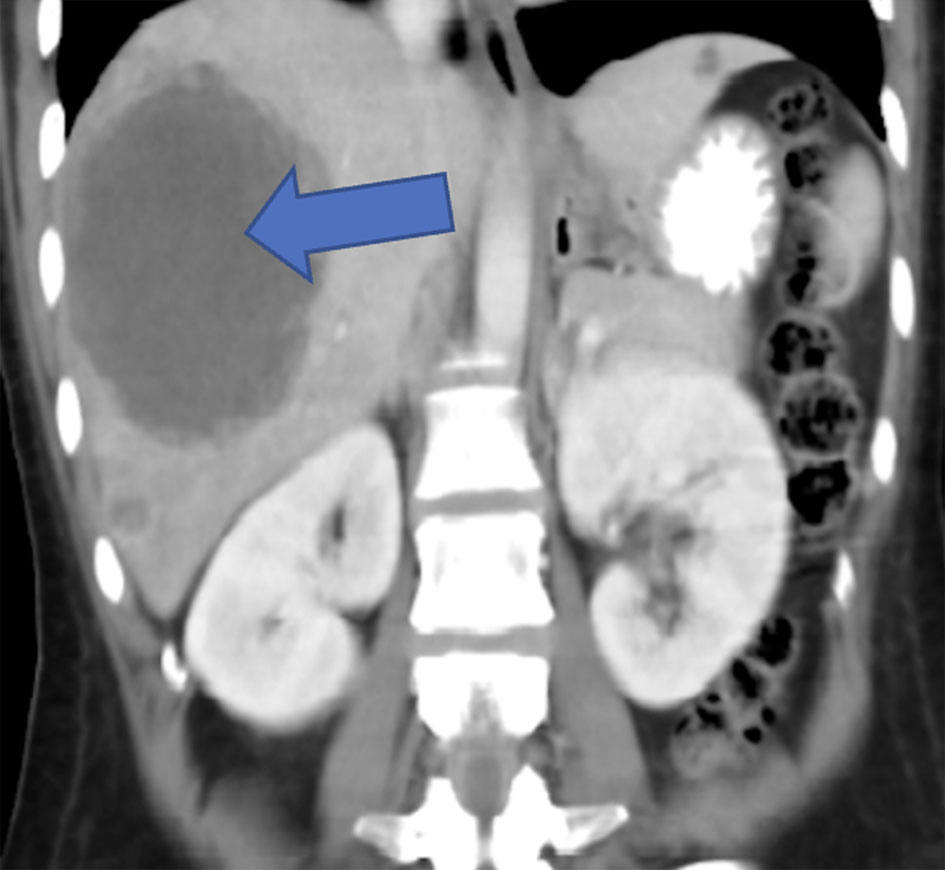 Click for large image | Figure 4. CT of abdomen and pelvis (2 months prior to presentation) showing multiple different sized cystic lesions, many of them with ring enhancement seen scattered in both lobes of the liver, with the largest measuring about 10 cm and located in segment VII (blue arrow). CT: computed tomography. |
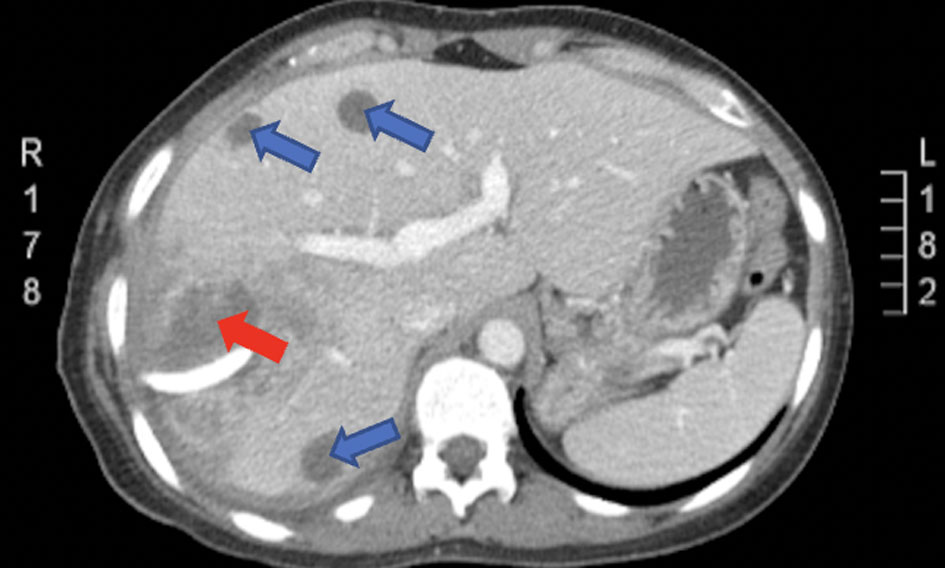 Click for large image | Figure 5. CT of abdomen and pelvis with IV contrast 2 months prior showing numerous cystic lesions (blue arrows) scattered in both lobes of the liver, with the largest measuring 12 × 10 cm compressing segment VII (red arrow). CT: computed tomography. |
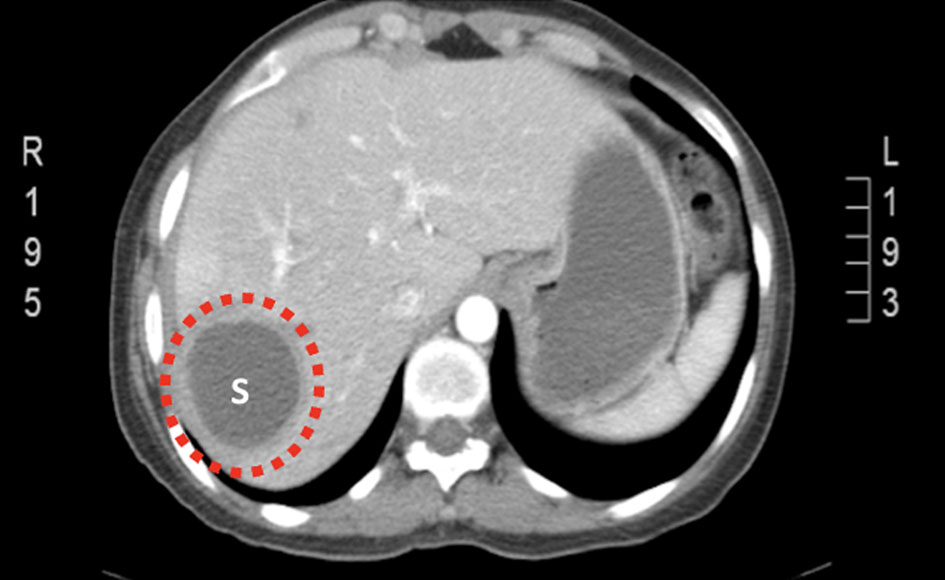 Click for large image | Figure 6. CT of abdomen and pelvis (on admission) showing abscess recurrence in segment VII (encircled). CT: computed tomography. |
Case 3
A 44-year-old male with no significant past medical history presented with fever, chills, rigors, right upper abdominal pain, and shortness of breath. Four weeks earlier, he had similar manifestations and was diagnosed with a right-sided pleural effusion and basal consolidation and treated conservatively with oral antibiotics. Labs were notable for leukocytosis of 15.7 × 103 cells/dL with left shift, CRP of 201, erythrocyte sedimentation rate (ESR) of 104 mm/h, albumin of 2.9, and ALP of 186 with negative blood cultures. CT of abdomen and pelvis showed a large liver abscess in segment VII measuring 5 × 6.1 cm and segment VI measuring 8.7 × 8 cm (Fig. 7). CT-guided drainage failed to improve the patient’s clinical condition; hence surgical intervention with a cholecystectomy with resection of segment VII and segment VI through a right subcostal abdominal incision was performed. The aspirated culture showed no growth. A follow-up ultrasound 1 week later showed no fluid accumulation and a CRP level of 147.
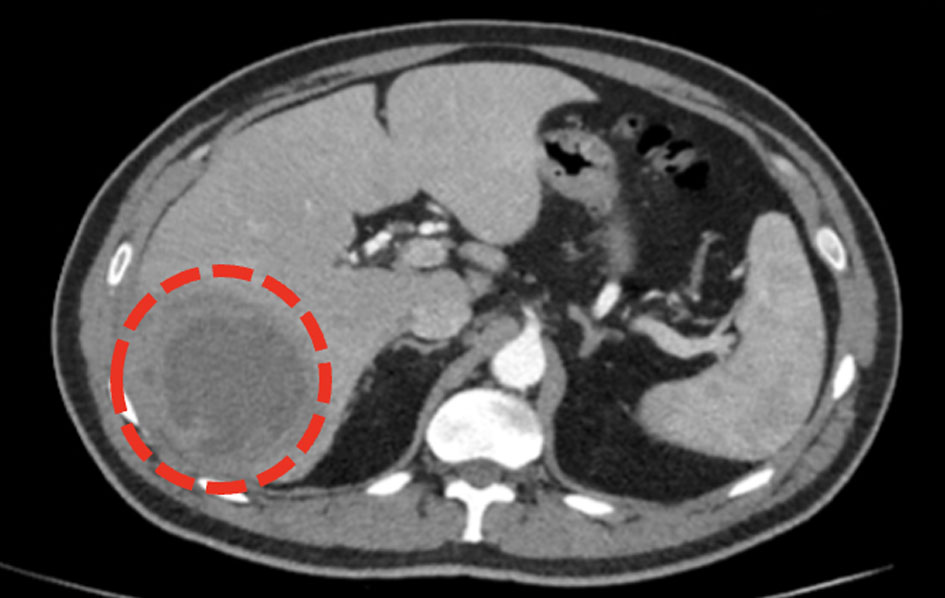 Click for large image | Figure 7. CT scan of abdomen and pelvis showing a large liver abscess in segment VII measuring 5 × 6.1 cm (encircled) and segment VI measuring 8.7 × 8 cm (not shown). CT: computed tomography. |
Case 4
A 22-year-old man with no significant medical history was admitted to our hospital 1 month after an explosion with shrapnel injuries to his right chest and upper right abdomen. At the time of the injury, the patient was admitted to a peripheral hospital in Yemen. He had sustained multiple shrapnel injuries with entry points in his right posterior chest, and exit points were in the right anterior axillary line at the level of the 10th rib. At that time, he underwent a laparotomy and was found to have extensive grade V liver injury of segments V, VII, and VIII with diaphragmatic perforation and primary lung injury. Extensive hemostasis was secured, and he was transferred to the intensive care unit (ICU) with a right chest drain and two abdominal drains (Fig. 8). During his follow-up course, he complained of severe persistent chest pain, progressive shortness of breath, and developed high-grade fever on several occasions. CT scans of the chest, abdomen, and pelvis with IV contrast showed liver space damage occupied with devitalized tissue and air-fluid levels, a right posterior diaphragmatic defect connecting the pleural space with the injured part of the liver, and multiple shrapnel fragments were seen scattered throughout the damaged liver space (Fig. 9). He was transferred to our hospital for further evaluation and management.
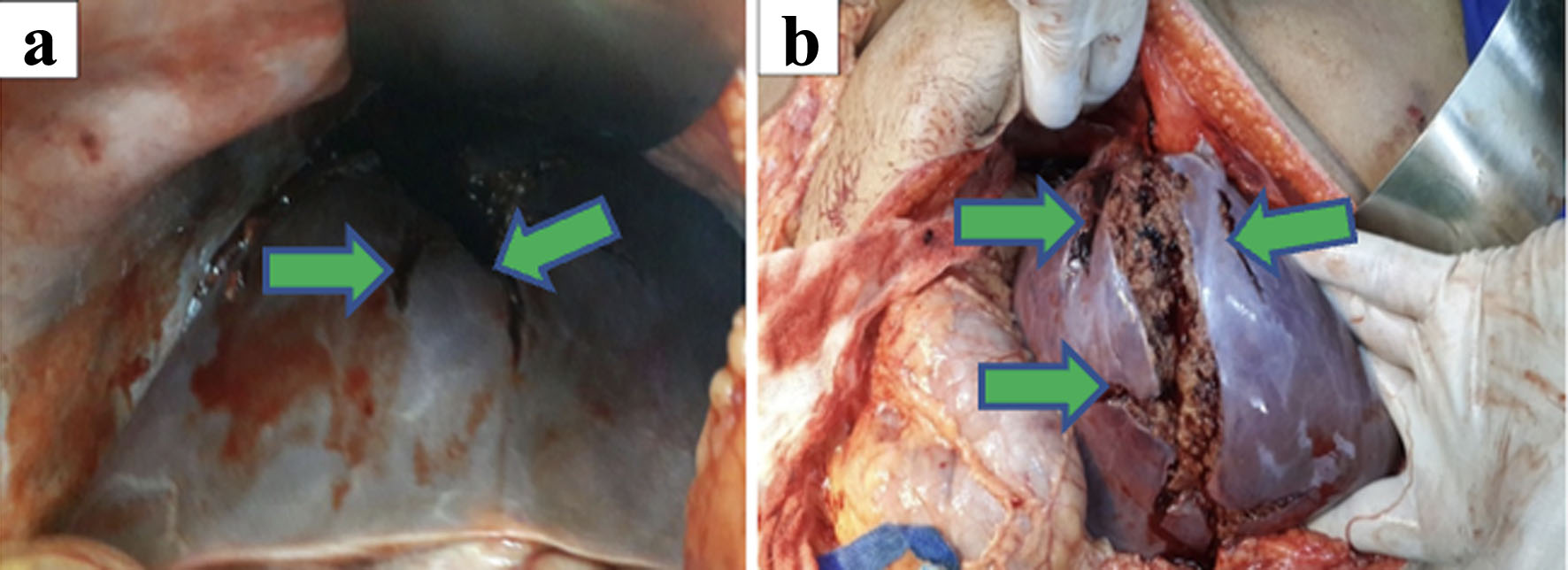 Click for large image | Figure 8. (a, b) Laparotomy findings of extensive grade V liver injury of segments V, VII, and VIII (green arrows) with diaphragmatic perforation. |
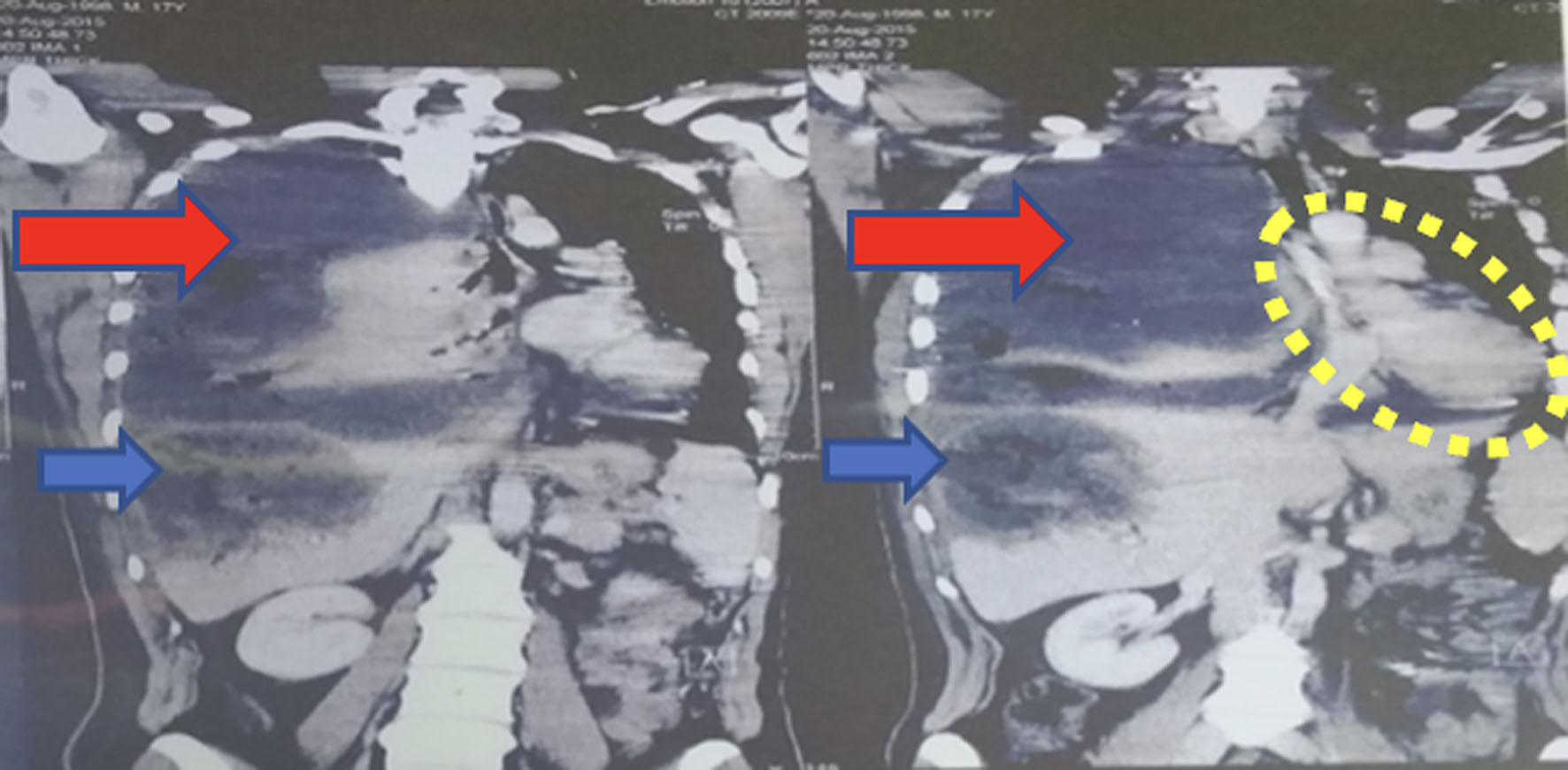 Click for large image | Figure 9. CT scan of abdomen and pelvis showing a massive fluid collection in the right pleural space (red arrows) herniated through the anterior mediastinum into the left side and displaced mediastinum (encircled), collapsed right lung, and large liver heamtoma (blue arrow). CT: computed tomography. |
On presentation to our facility, he complained of shortness of breath, right chest pain, and right upper abdominal pain. The abdomen was distended with diffuse tenderness and absent bowel sounds. His laboratory workup revealed leukocytosis of 15.2 × 103, CRP of 250, albumin of 3.7, and ALP of 332, along with negative blood cultures. CT-guided aspiration was performed with cultures positive for Pseudomonas aeruginosa. CT scans of the chest, abdomen and pelvis (Fig. 10) revealed an irregularity of the lateral aspect of the right hepatic lobe with multiple hypodensities associated with a large fluid collection of approximately 11 × 10 × 7 cm containing gas inclusions and small metal splinters, which indicated an infection hematoma. Ultrasound-guided drainage of the right liver abscess was performed, and the culture was positive for Pseudomonas aeruginosa. During his hospitalization, he had a persistent fever, and his laboratory tests revealed a total bilirubin of 1.5 with direct bilirubin of 0.93, white blood cell (WBC) of 12.8, and ALP of 332. An endoscopic retrograde cholangiopancreatography (ERCP) with a common bile duct (CBD) stent was performed, but it did not improve his clinical condition.
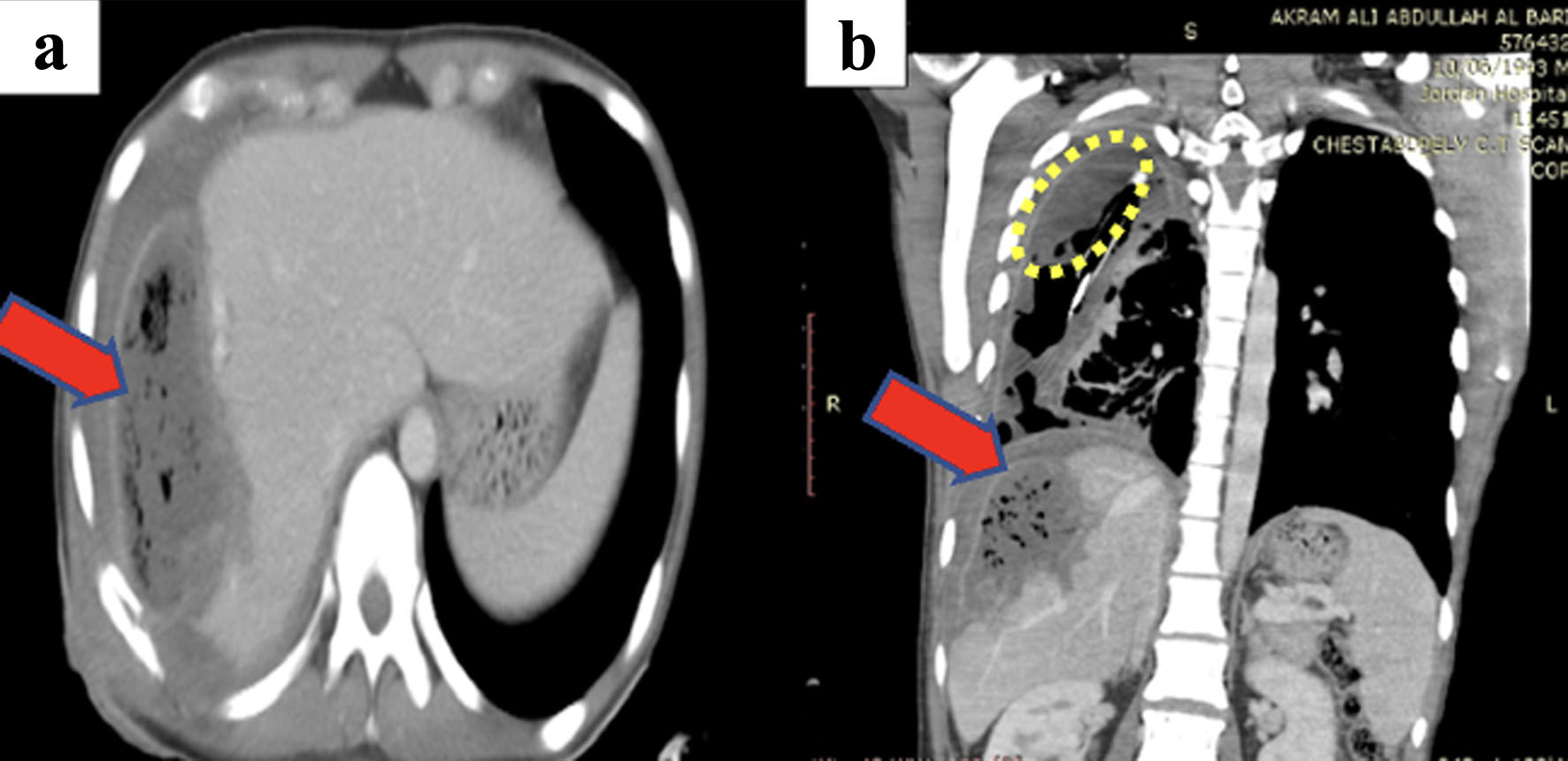 Click for large image | Figure 10. (a, b) CT scan of chest and abdomen showing the periphral enhancement of the pleural fluid collection (encircled) indicating empyema, multiple right-sided fluid loculations and partial right lung collapse noted with atelectasis. Right heaptic lobe shows large fluid collection with multiple air pockets (red arrow). CT: computed tomography. |
Repeated abdominal CT showed an increasing size of the liver abscess. The patient underwent right hepatectomy. During his postoperative course, he remained clinically stable, with his fever decreasing and his laboratory values improving. He was discharged on the eighth postoperative day. Follow-up ultrasound 1 week later showed no evidence of a liver abscess.
| Discussion | ▴Top |
Pyogenic abscesses account for almost 80% of all liver abscesses in developed countries and are most commonly polymicrobial [6]. Traditionally, treatment for liver abscesses has been open surgical treatment. However, percutaneous and laparoscopic drainage has gradually expanded [5]. Despite these innovative techniques and improved imaging, clinicians may face significant challenges in diagnosing and treating liver abscesses [6]. Surgical intervention remains a viable rescue option in certain situations [7]. Here we present four cases of PLA of different etiologies requiring partial liver resection to achieve a definitive cure. We highlight the risk factors, common pathogens, and treatment options (Table 1).
 Click to view | Table 1. Demographic and Clinical Profiles of the Four Cases |
The etiology of PLA can be divided into six categories: 1) biliary infection; 2) seeding of the portal vein (pylephlebitis); 3) direct spread; 4) seeding of the hepatic arteries; 5) penetrating trauma; and 6) cryptogenic cause [1]. The etiology of these abscesses has changed in the last decades. Historically, the most common cause was acute appendicitis. The series by Johns Hopkins, which first described these categories, reported a shift of appendicitis with transvenous spread as the leading cause of biliary obstruction, often caused by cholangiocarcinoma and particularly after palliative stenting [1]. Also, the increasing frequency of cholelithiasis and biliary tract pathologies, with their potential to induce ascending cholangitis, have increased biliary etiology as the leading cause of liver abscesses [8]. In our series, the underlying etiologies of liver abscesses were as follows: one traumatic from a shrapnel blast injury, one following trans-arterial chemoembolization in HCC, and two patients with no apparent etiology. In the first case, the patient had underlying risk factors such as liver cirrhosis, hepatitis B, and chemoembolization for HCC 2 years ago. The coexistence of malignancies in PLA is common, ranging from 16.2% in a general hospital to 86% in a specialized surgical center [9]. The association between radiofrequency ablation and transarterial chemoembolization and PLA has been described, and these procedures are associated with infections by skin flora, such as streptococci and staphylococci [9]. In the second case, the patient had an inflammatory cecal mass and recurrent liver abscesses, but we could not recognize the source of the infection. Recent reports have linked PLA to subclinical colon cancer. The associations appear to be more prominent in patients with Klebsiella infection, although whether all patients with PLA should undergo colonoscopy remains controversial [10]. In the third case, the patient had no significant past medical history, and the cause of PLA was unknown, with negative growth on cultures. The fourth case was challenging, with PLA attributed to a traumatic explosion and Pseudomonas growing on aspirated and intraoperative drainage cultures.
CT of abdomen with IV contrast is the preferred imaging modality for PLA with a sensitivity reaching 100% [9, 11]. A positive bacterial culture supports the radiographic diagnosis from peripheral blood or abscess aspirate [12]. However, over 30% of cases can be culture-negative [1]. In our series, CT of the abdomen and pelvis with IV contrast was used for diagnosis in all cases. Cultures from abscess aspirates grew E. coli with CT-guided drainage and Enterococcus faecium with open surgical drainage in the first case. In contrast, Pseudomonas aeruginosa grew with US-guided drainage aspirate culture in the fourth case. The second and third cases had negative cultures.
Combined with targeted antimicrobial therapy, percutaneous drainage techniques are now the mainstay of treatment. However, a small proportion of patients do not respond adequately to minimally invasive strategies, for which traditional open surgery is the definitive option [7]. Surgical options include surgical drainage or partial liver resection [13]. Surgical resection was considered in certain clinical circumstances: 1) Liver abscess not amenable to percutaneous drainages, such as huge, multiseptated, or multifocal abscesses [14]; 2) Ruptured liver abscess or fistula formation in which surgery is highly likely required [11]; 3) Failed treatment with an optimal antibiotic therapy and percutaneous drainage [15]; and 4) concomitant pathologies requiring surgical intervention, such as malignancy or biliary abnormality [15]. Depending on the surgeon’s experience and the location of the liver abscess, liver resection can be performed by (mini)laparotomy or a less invasive laparoscopic approach [15]. In the first case, open surgical abscess drainage with partial liver resection (segment VII resection) was necessary due to the failure of antibiotics and percutaneous aspiration. In the second case, a cholecystectomy with partial hepatic resection (resection of segment VII and most of segment VI) was required after two failed attempts at antibiotics in combination with CT-guided drainage. In the third case, the abscess was large and not amenable to percutaneous intervention, necessitating cholecystectomy with partial hepatic resection (segment VII and segment VI resection). Finally, in the fourth case, a right hepatectomy was required after failure of the US-guided drainage and clinical deterioration of the patient.
Conclusion
Percutaneous aspiration or drainage and aggressive antibiotic treatment have become the mainstays of treatment, replacing surgery as the first line of treatment. Under certain circumstances, surgical options, such as open drainage or liver resection, may offer a viable and curative alternative.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent to publish these cases, including images, was obtained from the four included patients.
Author Contributions
Aiman Obed, Mohammad Abuassi, and Saqr Alsakarneh wrote and revised the manuscript. Fouad Jaber, Mahmoud Fakhri, Fadi Abufares, Abdalla Bashir, Mahmood Syam, Anwar Jarrad, Ody Abdelhadi, and Hassan Ghoz participated in writing and revising the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article. Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
| References | ▴Top |
- Huang CJ, Pitt HA, Lipsett PA, Osterman FA, Jr., Lillemoe KD, Cameron JL, Zuidema GD. Pyogenic hepatic abscess. Changing trends over 42 years. Ann Surg. 1996;223(5):600-607; discussion 607-609.
doi pubmed pmc - Li W, Wu C, Qin M, Cai F, Huang J. The aura of malignant tumor: Clinical analysis of malignant tumor-related pyogenic liver abscess. Medicine (Baltimore). 2020;99(9):e19282.
doi pubmed pmc - Serraino C, Elia C, Bracco C, Rinaldi G, Pomero F, Silvestri A, Melchio R, et al. Characteristics and management of pyogenic liver abscess: A European experience. Medicine (Baltimore). 2018;97(19):e0628.
doi pubmed pmc - Cai YL, Xiong XZ, Lu J, Cheng Y, Yang C, Lin YX, Zhang J, et al. Percutaneous needle aspiration versus catheter drainage in the management of liver abscess: a systematic review and meta-analysis. HPB (Oxford). 2015;17(3):195-201.
doi pubmed pmc - Ahmed M, Alam J, Hussain S, Aslam M. Prospective randomized comparative study of percutaneous catheter drainage and percutaneous needle aspiration in the treatment of liver abscess. ANZ J Surg. 2021;91(3):E86-E90.
doi pubmed - Mischnik A, Kern WV, Thimme R. [Pyogenic liver abscess: changes of organisms and consequences for diagnosis and therapy]. Dtsch Med Wochenschr. 2017;142(14):1067-1074.
doi pubmed - Santos-Rosa OM, Lunardelli HS, Ribeiro-Junior MA. Pyogenic liver abscess: diagnostic and therapeutic management. Arq Bras Cir Dig. 2016;29(3):194-197.
doi pubmed pmc - Branum GD, Tyson GS, Branum MA, Meyers WC. Hepatic abscess. Changes in etiology, diagnosis, and management. Ann Surg. 1990;212(6):655-662.
doi pubmed pmc - Mezhir JJ, Fong Y, Jacks LM, Getrajdman GI, Brody LA, Covey AM, Thornton RH, et al. Current management of pyogenic liver abscess: surgery is now second-line treatment. J Am Coll Surg. 2010;210(6):975-983.
doi pubmed - Siu LK, Yeh KM, Lin JC, Fung CP, Chang FY. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect Dis. 2012;12(11):881-887.
doi pubmed - Alvarez Perez JA, Gonzalez JJ, Baldonedo RF, Sanz L, Carreno G, Junco A, Rodriguez JI, et al. Clinical course, treatment, and multivariate analysis of risk factors for pyogenic liver abscess. Am J Surg. 2001;181(2):177-186.
doi pubmed - Johannsen EC, Sifri CD, Madoff LC. Pyogenic liver abscesses. Infect Dis Clin North Am. 2000;14(3):547-563.
doi pubmed - Alkofer B, Dufay C, Parienti JJ, Lepennec V, Dargere S, Chiche L. Are pyogenic liver abscesses still a surgical concern? A Western experience. HPB Surg. 2012;2012:316013.
doi pubmed pmc - Chung YF, Tan YM, Lui HF, Tay KH, Lo RH, Kurup A, Tan BH. Management of pyogenic liver abscesses - percutaneous or open drainage? Singapore Med J. 2007;48(12):1158-1165.
pubmed - Heneghan HM, Healy NA, Martin ST, Ryan RS, Nolan N, Traynor O, Waldron R. Modern management of pyogenic hepatic abscess: a case series and review of the literature. BMC Res Notes. 2011;4:80.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


