| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Original Article
Volume 16, Number 2, April 2023, pages 92-95
Quality of Capsule Endoscopy Reporting in Patients Referred for Double Balloon Enteroscopy
Joshua Leea, c , Jonathan Reichsteinb, Iris Vancea, Daniel Wilda
aDivision of Gastroenterology, Duke University Medical Center, Durham, NC, USA
bDepartment of Internal Medicine, Duke University Medical Center, Durham, NC, USA
cCorresponding Author: Joshua Lee, Division of Gastroenterology, Duke University Medical Center, Durham, NC 27710, USA
Manuscript submitted January 3, 2023, accepted March 27, 2023, published online April 28, 2023
Short title: Quality of Capsule Endoscopy Reporting
doi: https://doi.org/10.14740/gr1596
| Abstract | ▴Top |
Background: Abnormal video capsule endoscopy (VCE) findings often require intervention with double balloon enteroscopy (DBE). Accurate VCE reporting is important for procedural planning. In 2017 the American Gastroenterological Association (AGA) published a guideline that included recommended elements for VCE reporting. The aim of this study was to examine adherence to the AGA reporting guidelines for VCE.
Methods: The medical records of all patients who underwent DBE at a tertiary academic center between February 1, 2018, and July 1, 2019, were retrospectively reviewed to identify the VCE report that prompted DBE. Data were collected on the presence of each reporting element recommended by the AGA. Differences in reporting between academic and private practices were compared.
Results: A total of 129 VCE reports were reviewed (84 private practice and 45 academic practice). Reports consistently included indication, date, endoscopist, findings, diagnosis, and management recommendations. Timing of anatomic landmarks and abnormalities were included in only 87.6% of reports and preparation quality in only 26.2%. Reports from private practice groups were significantly more likely to include the type of capsule (P < 0.001). VCE reports from academic centers were more likely to include adverse outcomes (P < 0.001), pertinent negatives (P = 0.0015), extent of exam (P = 0.009), previous investigations (P = 0.045), medications (P < 0.001), and document communication to patient/referring physician (P = 0.001).
Conclusions: Most VCE reports in both private and academic settings included the important elements recommended by the AGA; however only 87% listed the times of landmarks and abnormal findings, which are crucial in determining the type and direction of approach for subsequent interventions. It is unclear whether the quality of VCE reporting influences the outcome of subsequent DBE.
Keywords: Video capsule endoscopy; Endoscopy reporting; Quality improvement; Guideline adherence
| Introduction | ▴Top |
Since its approval in 2001, video capsule endoscopy (VCE) has been a widely used and important technique for small bowel evaluation [1]. Positive VCE findings often require subsequent intervention with device-assisted enteroscopy (DAE). The decision to pursue DAE, the modality used (push enteroscopy vs. single balloon vs. spiral vs. double balloon) and the direction of approach (antegrade or retrograde) are chosen based largely upon the expected location of the small bowel findings [2]. Since DAE is often performed at referral centers separate from where the original VCE was performed, the quality of VCE reporting is crucial. Though abnormal VCE studies are ideally reviewed by enteroscopists prior to DAE, the technical limitations of large data transfers do not always allow for this to occur in a timely fashion, making the quality of reporting that much more important. Recognizing the importance of this documentation, the American Gastroenterological Association (AGA) adapted the Canadian consensus guidelines of endoscopy reporting and applied them for the first time directly to VCE in 2017 (Table 1) [1, 3]. Despite these recommendations and its clinical importance, VCE reporting remains inconsistent. This study aimed to examine the quality of VCE reporting and adherence to these guidelines in both private practice and academic settings.
 Click to view | Table 1. Report Variables Based on Canadian Consensus Guidelines of Endoscopy Reporting as Adapted by the AGA (2017) |
| Materials and Methods | ▴Top |
Patient population
After receiving the Institutional Review Board (IRB) approval, an internally maintained database and scheduling records were used to identify all patients who underwent double balloon enteroscopy (DBE) at a single large academic referral center between February 1, 2018, and July 1, 2019. Charts were then retrospectively reviewed to analyze the VCE report that prompted referral for DBE. All capsules performed at our academic institution and at the majority of external referring practices were completed using Medtronic PillCam™. The study was conducted in compliance with the ethical standards of the responsible institution and under the Duke IRB approval (Pro00103152).
Inclusion criteria
Patients were included in the study if they had undergone DBE at our medical center during our study period and had a VCE report available within the electronic medical record. Patients with multiple capsule reports were only included twice if there was a second DBE associated with the second VCE.
Exclusion criteria
Patients were excluded from the study if they did not have a formal VCE report within the electronic medical record.
Data gathering and storage
Data were retrospectively extracted from our electronic medical record. Both internal capsule reports and scanned reports from outside facilities were reviewed if available. Specific data points captured in the study included the presence or absence of the following reporting variables that are recommended by the AGA (Table 1). The type of practice in which the report was created, academic center or private practice, were also recorded.
Data analysis
The percentage of inclusion for each reporting variable was recorded. VCE reports were then stratified based upon whether they were performed in an academic or private practice setting. The difference between these percentages was analyzed using χ2 test for proportions. The null hypothesis is that the difference in proportions between each element is zero. An α < 0.05 was used for significance to reject the null hypothesis.
| Results | ▴Top |
A total of 209 DBEs were performed during our investigated time span. Of those, 68 patients had no formal VCE report available within the electronic medical record and 12 patients had repeat DBE without repeat VCE, leaving 129 VCE reports for analysis (Fig. 1). Of these, 84 were from private practice setting and 45 were from academic centers.
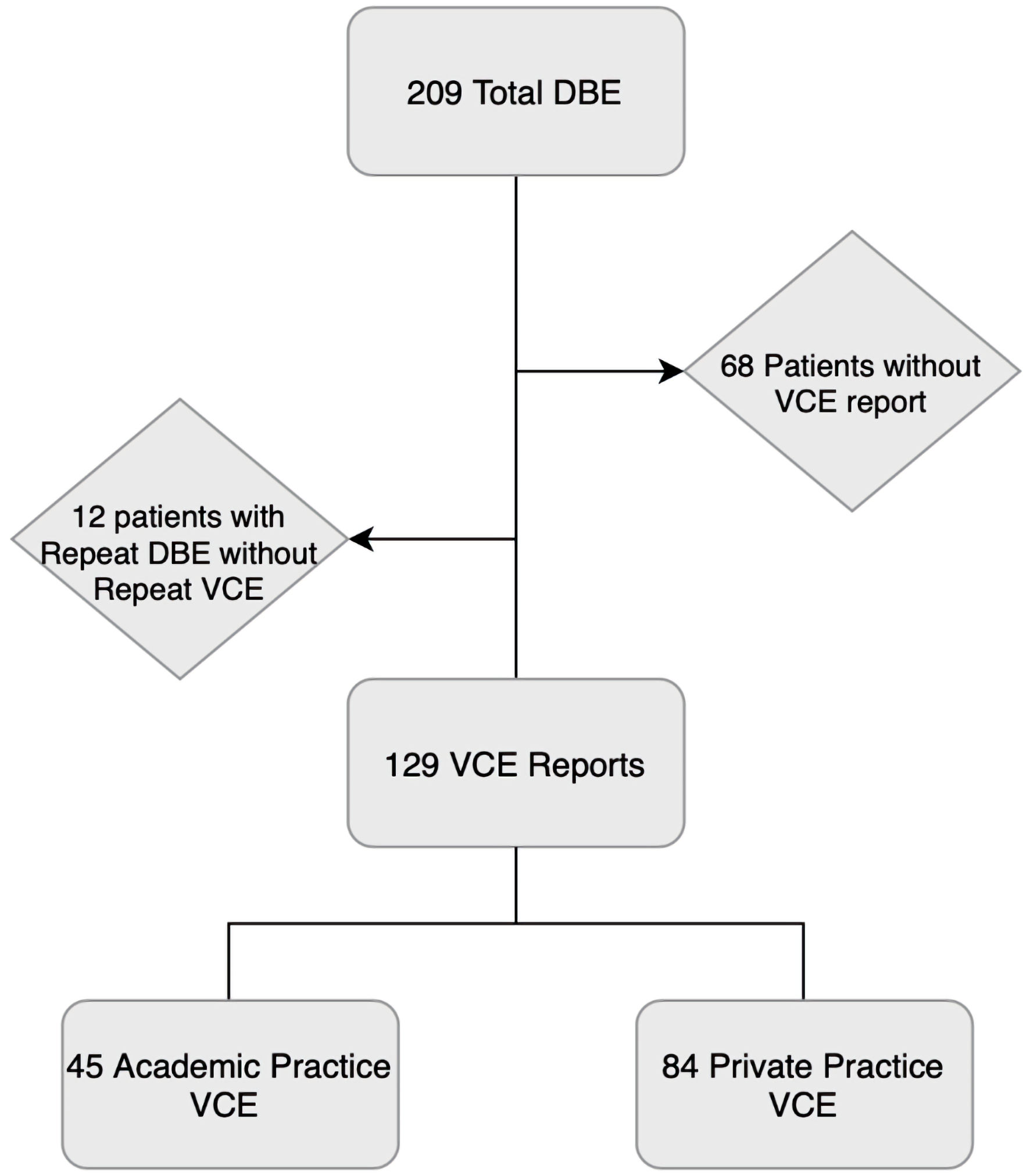 Click for large image | Figure 1. Flowsheet of final capsule report count. A total of 209 DBEs were performed. Of those, 68 patients had no capsule report in the electronic medical record and 12 patients had repeat DBE without repeat VCE. One patient who had repeat DBE had a private practice VCE and a second VCE at an academic center prior to second DBE. A total of 129 capsule reports were analyzed, 45 academic practice and 84 private practice. VCE: video capsule endoscopy; DBE: double balloon enteroscopy. |
The combined results of all capsule reports are shown in Table 2. Reports consistently included indication (100%), date (100%), name of reader (96.9%), findings (98.4%), diagnosis (92.2%) and management recommendations (92.2%). Anatomic landmark (stomach, duodenum, and cecum) and abnormality times were included in only 87% of reports. Reports rarely noted the type of bowel preparation (3.8%), quality of preparation (23.2%), patient allergies (0%) or the presence or absence of adverse events (16.2%) and rarely documented whether findings were communicated to another provider or the patient (20.9%).
 Click to view | Table 2. Presence of Report Variables Among VCE Reports |
The results of the capsule reports stratified by type of practice creating the report are shown in Table 2. Reports from private practice groups were significantly more likely to include the type of capsule system (86.9% vs. 40.0%, P < 0.001). VCE reports from academic institutions were more likely to include the presence or absence of adverse outcomes (42.2% vs. 2.4%, P < 0.001), pertinent negatives (77.8% vs. 48.8%, P = 0.002), and to document whether or not the exam was complete (reached the cecum) (88.9% vs. 67.9%, P = 0.009). Academic centers were also more likely to document post-procedure communication with the patient or referring provider(s) (37.8% vs. 11.9%, P = 0.001), list previous investigations (57.8% vs. 39.3%, P = 0.045) and detail any medications administered during the procedure (55.6% vs. 8.3%, P = 0.001). There were no significant differences in reporting key anatomic landmark times, findings, diagnosis, and management recommendations between the two groups. Both practice settings had very low reporting rates of patient allergies and quality of bowel preparation.
| Discussion | ▴Top |
Though its direct impact on clinical care remains uncertain, documentation is an important part of clinical practice. This is especially true for VCE, where decisions on whether to perform subsequent DAE and what modality and direction of approach to pursue are often based largely on documented VCE findings. In this study, we sought to analyze the quality of VCE reporting for patients referred for DBE at a single tertiary care center and investigate whether reporting quality varied significantly between the type of referring practice.
It is reassuring that the majority of reports in both clinical settings featured the many important and clinically useful elements including the name of the capsule reader, procedure indication, key findings, and management recommendations. We note, however, that despite their importance in determining the modality and direction of approach for DAE [2], only 87% of reports included the time of anatomic landmarks and the timing of any abnormal findings. With no clear way to distinguish segments of small bowel by their endoscopic appearance, the timing of a finding in relation to the capsule’s passage into the duodenum or its entering the cecum is the best way to estimate its location [2]. Since VCE studies are not always available or obtainable for direct review if performed at an outside office or facility, these reporting deficiencies may result in delayed care and increased cost by necessitating repeat VCE procedures or worse, may result in selecting the wrong DAE modality or direction of approach for subsequent interventions.
The use of a bowel preparation prior to VCE remains controversial. Though some studies suggest no benefit, a systemic review of 15 studies showed that the use of bowel cleansing with polyethylene glycol (PEG) resulted in significantly improved bowel visualization and diagnostic yield [4, 5]. Whether a preparation is used or not, the quality of the preparation has important clinical implications. The diagnostic yield of DBE performed after a positive VCE is much higher (75%) than when performed following a negative VCE (25%) [6]. Thus, a negative VCE is likely to dampen enthusiasm to pursue subsequent DAE unless the negative exam is in the setting of a poor or sub-optimal preparation. Despite its clinical implications and recommendation in the AGA guideline, only 23% of VCE reports across both practice types commented on preparation quality.
Though our study is limited by including patients referred to only a single tertiary academic center in the southeastern United States, we benefit from being the only center to provide DBE in a several hundred-mile radius. Therefore, the patients included in our study were referred from a wide range of practices and locations, ranging from small rural private practices to large urban hospitals. The retrospective nature of this study meant that all VCE reports were not available for review, which we recognize as another limitation.
We hope that these findings improve the quality of VCE reporting and lead to more wide-spread adoption of the AGA VCE reporting guidelines. Some of the reporting variance seems to stem from the type of program used to create the report, so we hope our findings lead to the creation of improved VCE reporting software which prompts providers to detail the key elements recommended in the guideline.
Further studies will also need to be done to determine whether the quality of VCE reporting has any bearing on the yield or outcome of subsequent DAE.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
All authors have no conflict of interest to disclose.
Informed Consent
No informed consent was needed for this study. IRB approval was obtained through the Duke IRB to conduct this retrospective study (Pro00103152).
Author Contributions
Generation of study idea: DW, IV, JL. Data collection: JL, JR. Data analysis: JL, DW. Data interpretation: all authors. Manuscript writing: JL, DW, JR. Manuscript review, critique, and revision: all authors.
Data Availability
The data supporting the findings of the study are available from the corresponding author upon reasonable request.
Abbreviations
VCE: video capsule endoscopy; DBE: double balloon enteroscopy; DAE: device-assisted enteroscopy
| References | ▴Top |
- Enns RA, Hookey L, Armstrong D, Bernstein CN, Heitman SJ, Teshima C, Leontiadis GI, et al. Clinical practice guidelines for the use of video capsule endoscopy. Gastroenterology. 2017;152(3):497-514.
doi pubmed - Chalazan B, Gostout CJ, Song LM, Enders FT, Rajan E. Use of capsule small bowel transit time to determine the optimal enteroscopy approach. Gastroenterology Res. 2012;5(2):39-44.
doi pubmed pmc - Armstrong D, Barkun A, Bridges R, Carter R, de Gara C, Dube C, Enns R, et al. Canadian Association of Gastroenterology consensus guidelines on safety and quality indicators in endoscopy. Can J Gastroenterol. 2012;26(1):17-31.
doi pubmed pmc - Hookey L, Louw J, Wiepjes M, Rubinger N, Van Weyenberg S, Day AG, Paterson W. Lack of benefit of active preparation compared with a clear fluid-only diet in small-bowel visualization for video capsule endoscopy: results of a randomized, blinded, controlled trial. Gastrointest Endosc. 2017;85(1):187-193.
doi pubmed - Kotwal VS, Attar BM, Gupta S, Agarwal R. Should bowel preparation, antifoaming agents, or prokinetics be used before video capsule endoscopy? A systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2014;26(2):137-145.
doi pubmed - Teshima CW, Kuipers EJ, van Zanten SV, Mensink PB. Double balloon enteroscopy and capsule endoscopy for obscure gastrointestinal bleeding: an updated meta-analysis. J Gastroenterol Hepatol. 2011;26(5):796-801.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


