| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Original Article
Volume 15, Number 6, December 2022, pages 353-363
Clinical Features and Management of Small Bowel Masses Detected During Device-Assisted Enteroscopy: A Multi-Center Experience
Michael G. Noujaima, e, Claire Dorseya, Alice Parishb, Daniel Rainesc, Lara Boudreauxc, Mark Hanscomd, David Caved, Donna Niedzwieckib, Daniel Wilda
aDivision of Gastroenterology, Duke University Medical Center, Durham, NC, USA
bDepartment of Biostatistics and Bioinformatics, Duke University, Durham, NC, USA
cDivision of Gastroenterology, Louisiana State University Health Sciences Center, New Orleans, LA, USA
dDivision of Gastroenterology, University of Massachusetts Medical School, Worcester, MA, USA
eCorresponding Author: Michael G. Noujaim, Division of Gastroenterology, Duke University Medical Center, Durham, NC, USA
Manuscript submitted October 29, 2022, accepted December 8, 2022, published online December 18, 2022
Short title: Clinical Features and Management of SBMLs
doi: https://doi.org/10.14740/gr1586
| Abstract | ▴Top |
Background: Small bowel mass lesions (SBMLs) are rare, span a range of different histologies and phenotypes, and our understanding of them is limited. Some lesions occur in patients with recognized polyposis syndromes and others arise sporadically. The current literature regarding SBMLs is limited to small retrospective studies, case reports, and small case series. This large multi-center study aims to understand the various clinical presentations, histologies and management options for SBMLs.
Methods: After obtaining Institutional Review Board (IRB) approval, electronic records were used to identify all device-assisted enteroscopy (DAE) performed for luminal small bowel evaluation in adult patients at three US referral centers (Duke, LSU and UMass) from January 1, 2014, to October 1, 2020. We identified all patients within this cohort in whom a SBML was detected. Using a focused electronic medical record chart review, we collected patient, procedure, and lesion-related data and used descriptive statistics to explore relationships between these data and outcomes.
Results: A total of 218 patients (49 at Duke, 148 at LSU, and 21 at UMass) in this cohort had at least one SBML found on DAE. The most common presenting symptoms were iron-deficiency anemia/bleeding (73.3%) and abnormal imaging (33.6%). Thirty-five percent of patients had symptoms for more than a year prior to their diagnosis. Most patients (71.6%) underwent video capsule endoscopy (VCE) prior to DAE and 84% of these exams showed the lesion. The lesion was seen less frequently (48.9%) on computed tomography (CT) scan performed prior to DAE. The majority of lesions were found on antegrade (56%) or retrograde (29.8%) double-balloon enteroscopy (DBE). The most common lesion phenotypes were submucosal (41.3%) and pedunculated (33%) with a much smaller number being sessile (14.7%) or obstructing/invasive (11%). They were found equally as commonly in the jejunum (46.3%) and ileum (49.5%). Most lesions were 10 - 20 mm in size (47%) but 22.1% were larger than 20 mm. The most common histologies were neuroendocrine tumors (NETs, 20.6%) and hamartomas (20.6%). Primary adenocarcinoma of the small bowel was rare, constituting only 5% of lesions. The majority of polyps (78.4%) were sporadic, compared to 21.7% associated with a polyposis or hereditary cancer syndrome, most commonly Peutz-Jeghers syndrome (18.3%). After DAE, 37.6% were advised to undergo surgical resection and 48% were advised to undergo endoscopic surveillance or no further management because of benign histology or successful endoscopic resection.
Conclusions: In this multi-center retrospective study we found that SBMLs are more likely to be sporadic than syndromic, medium in size and either pedunculated or submucosal. NETs and hamartomas predominated and symptoms, most commonly anemia, can be present for more than a year prior to diagnosis. Close to one half of lesions required either no further intervention or only endoscopic surveillance.
Keywords: Small bowel mass lesions; Device-assisted enteroscopy; Tumors of the small intestine; Video capsule endoscopy; Iron-deficiency anemia; Gastrointestinal bleeding
| Introduction | ▴Top |
Although the small bowel constitutes the majority of the gastrointestinal (GI) tract, it accounts for only 3-6% of GI mass lesions or tumors and 0.6% of all new cancer cases in the United States each year [1, 2]. Small bowel mass lesions (SBMLs) span a range of histologies from benign to malignant and phenotypes from pedunculated polyps to submucosal masses [2]. Some lesions occur in patients with recognized polyposis syndromes and others arise sporadically [1]. Depending on their location, size, and histopathologic features these tumors can have widely variable and often insidious clinical presentations [1]. Therefore, the timely and accurate diagnosis and localization of SBMLs is crucial. Nevertheless, the diagnosis of SBMLs is difficult owing to their low incidence, nonspecific clinical presentation, and location beyond the reach of standard endoscopic evaluation [1, 3]. Indeed, up until the past two decades, these lesions were typically only diagnosed with surgical laparotomy [4, 5]. Since its approval in 2001, video capsule endoscopy (VCE) has played a significant role in detecting SBMLs in patients presenting with a variety of complaints ranging from occult GI bleeding to abdominal pain and weight loss. As it typically allows for inspection of the entire small bowel and has a diagnostic yield as high as 91% for detecting SBMLs, the incidence of detected SBMLs increased from 11.8 cases per million in 1973 to 22.7 cases per million in 2004 [3-9]. Though VCE has become the preferred first-line method for luminal small bowel evaluation, it is limited by its inability to provide a tissue diagnosis [1, 3, 8, 10]. To this end, the introduction of device-assisted enteroscopy (DAE) in 2000 provided a non-surgical, endoscopic modality that allowed for the direct examination of the entire small bowel [11, 12]. Currently, there are three main DAE modalities: double-balloon enteroscopy (DBE), single-balloon enteroscopy (SBE) and spiral enteroscopy (SE). DAE not only enables the direct visualization of the entire small bowel lumen, but also allows for the controlled sampling or complete removal of SBMLs [1, 8]. We recently published a large multi-center study on the use, risk, and yield of DAE across all three modalities that showed an overall diagnostic yield of 76.3% and 14% of the cohort had a SBML [8]. Other studies have shown the diagnostic yield of DBE for small bowel polyps to be as high as 100% [10].
The current literature regarding SBMLs is limited to small retrospective studies, case reports, and small case series. Here we present a large, retrospective, multi-center US study of 218 patients with SBMLs aiming to determine important clinical features of SBMLs including their most common presenting symptoms and how long those exist prior to diagnosis; their most common phenotypic features and histologies and whether they occur more commonly sporadically or in association with hereditary polyposis syndromes. We also sought to determine the efficacy of other diagnostic modalities at diagnosing SBMLs prior to DAE and to understand the optimal management approach after detection.
| Materials and Methods | ▴Top |
Study design and patient population
After obtaining Institutional Review Board (IRB) approval, electronic records were used to identify all DAEs performed for luminal small bowel evaluation in adult patients at three US referral centers (Duke, LSU and UMass) from January 1, 2014, to October 1, 2020. We identified all patients within this cohort in whom a SBML was detected. Using a focused electronic medical record chart review, we collected patient, procedure, and lesion-related data.
Statistical analysis
Patient, procedure, and lesion characteristics were summarized using frequency and percent for categorical variables, and median, first and third quartile for continuous characteristics. Associations between patient characteristics and histology types were assessed using Kruskall Wallis, Chi-square, or Fisher’s exact test, as appropriate. All analyses were conducted using SAS Software, version 9.4 (SAS Institute, Cary, NC).
Ethical approval
This retrospective chart review study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Human Investigation Committee (IRB) of Duke University approved this study on January 25, 2021.
| Results | ▴Top |
Patient characteristics and presenting symptoms
A total of 218 patients had a SBML detected on DAE at one of the three participating sites within our study period. The median age of patients with a SBML was 63 years old (range 18 - 89) and 54.1% were male (Table 1). The majority of patients (82.5%) had no family history of GI cancer and the majority of SBMLs (78.4%) were sporadic. Forty-six patients (21.2%) had a known hereditary cancer or polyposis syndrome, most commonly Peutz-Jeghers syndrome (PJS) (87.0%). Most patients were Caucasian (68.4%), non-smokers (66.1%) with a body mass index (BMI) between 25 and 39.9 kg/m2 (61.0%) at the time of diagnosis. Thirty-four point four percent of patients had symptoms for over 1 year at the time of presentation. Regardless of its histology, the majority of lesions presented with iron-deficiency anemia (IDA)/GI bleeding (73.4%) and/or abnormal imaging (including VCE) (33.5%). Lesions rarely (3.3%) presented with obstruction and other symptoms including abdominal pain (12%), diarrhea (11.1%), and weight loss (3.7%) were uncommon (Table 2).
 Click to view | Table 1. Patient Characteristics |
 Click to view | Table 2. Presenting Symptoms |
Imaging and endoscopy findings
All of the patients in this study were initially evaluated with at least one of the imaging and/or endoscopic modalities listed in Table 3 prior to undergoing the DAE that ultimately diagnosed the SBML. Most patients (71.6%) were evaluated with VCE and when compared to the other initial diagnostic modalities, VCE had the highest rates of SBML detection (84.6%). Cross-sectional imaging was employed less frequently prior to DAE and the rates of lesion detection of computed tomography (CT), computed tomography enterography (CTE) and magnetic resonance enterography (MRE) were 49%, 67% and 50% respectively. The majority of lesions were ultimately found on antegrade (56.0%) or retrograde (29.8%) DBE and SE and SBE were utilized much less often in these evaluations. Lesions detected on DAE frequently correlated with initial imaging or VCE findings (84% vs. 61% vs. 49% for VCE, CTE, and CT, respectively).
 Click to view | Table 3. Prior Diagnostic Studies and Correlation With DAE Findings |
Lesion characteristics
As expected in a study involving lesions diagnosed by DAE, the vast majority (95.8%) of SBML’s were found in the mid-to-distal small bowel with jejunal and ileal lesions being close to equal. Almost half of all lesions (47%) were 1 - 2 cm in size and the most common lesion phenotypes were submucosal (41.3%) and pedunculated (33.0%). Benign and malignant epithelial polyps/tumors constituted 37.2% and 28.4% of all histologies, respectively. More specifically, when further subclassified, neuroendocrine tumors (NETs, 20.6%) and hamartomas (20.6%) were the most common histologies. Patients with hamartomas were diagnosed at a significantly younger median age, 36.5, than patients with NETs, 65.5 (P < 0.001). African-American patients were significantly less likely to have hamartomas, 9%, than SBML’s of other histologies, 23-43% (P < 0.002) (Tables 4, 5 and Figs. 1-3).
 Click to view | Table 4. Lesion Characteristics |
 Click to view | Table 5. Descriptive Characteristics by Histology |
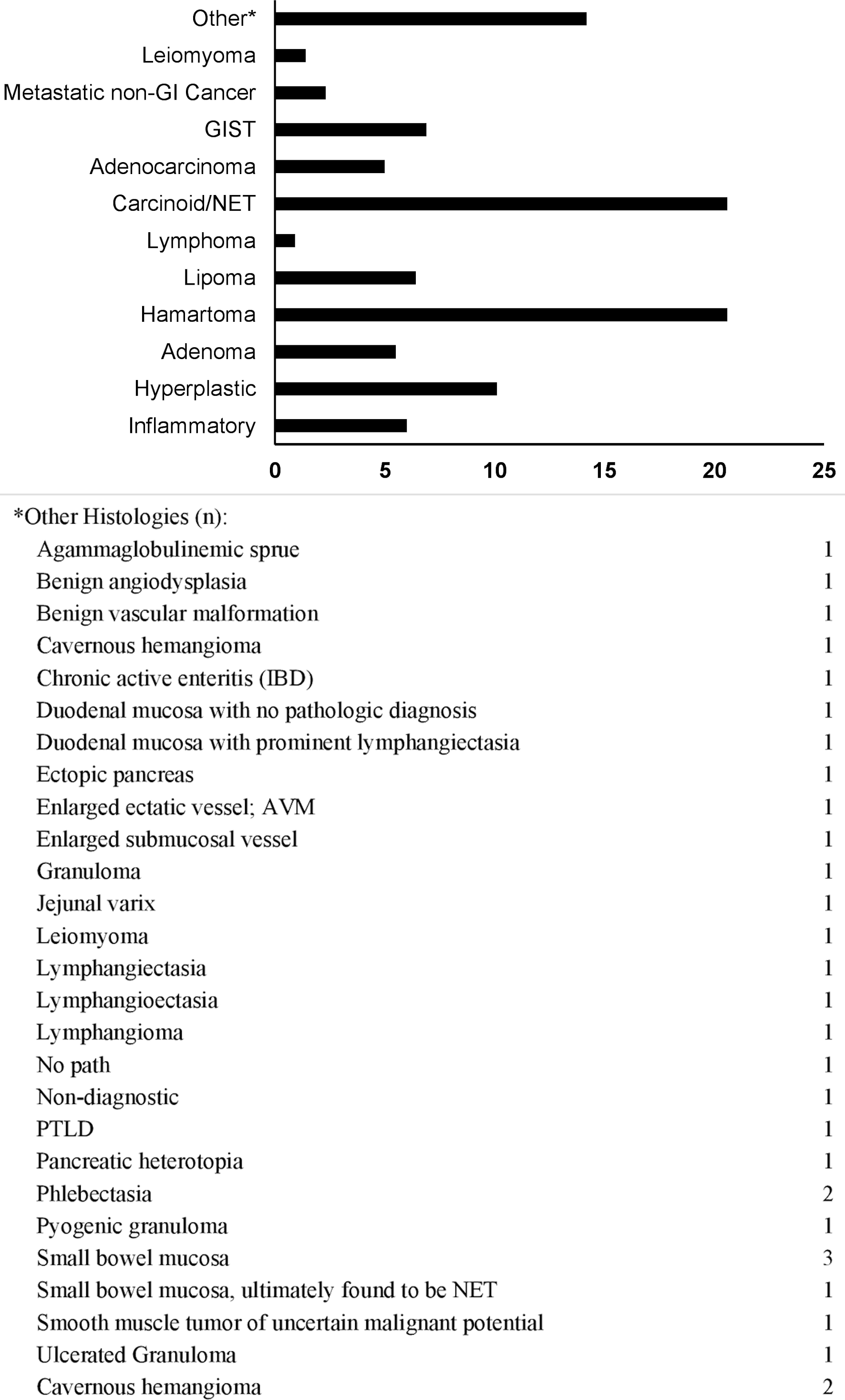 Click for large image | Figure 1. Lesion histology frequency (%). GIST: gastrointestinal stromal tumor; NET: neuroendocrine tumor; GI: gastrointestinal. |
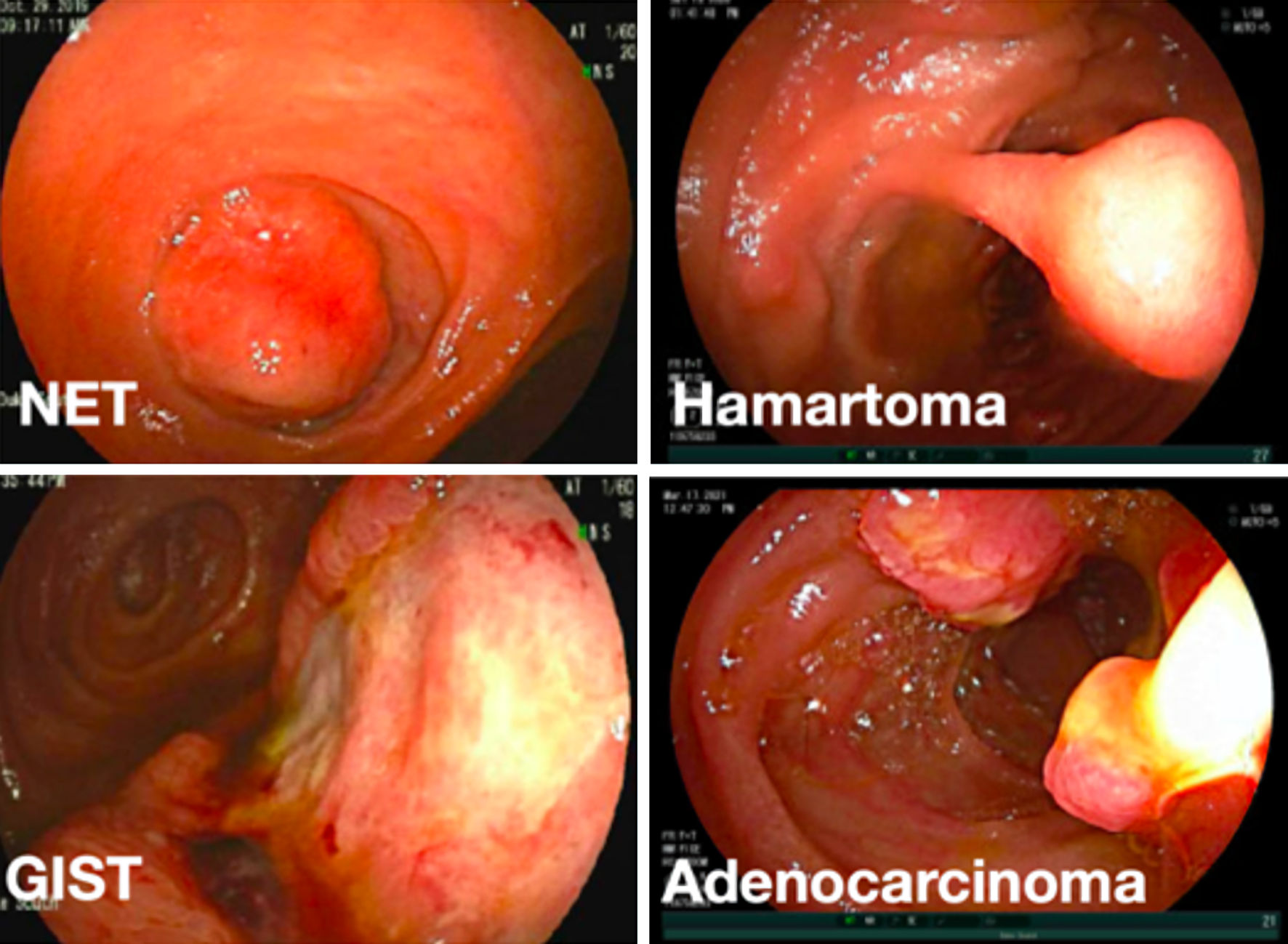 Click for large image | Figure 2. Representative endoscopic images of different small bowel mass lesions detected on device-assisted enteroscopic exams. NET: neuroendocrine tumor; GIST: gastrointestinal stromal tumor. |
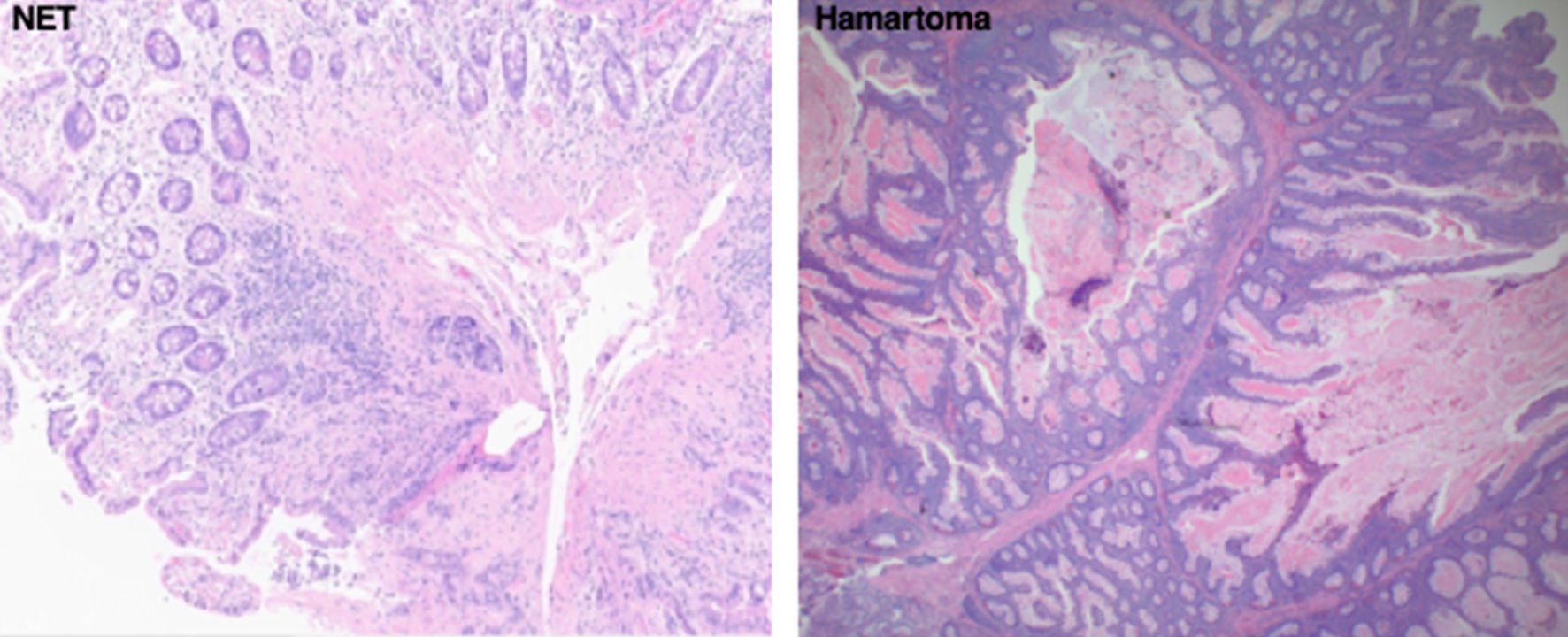 Click for large image | Figure 3. Representative biopsy histologic slides of the two most common small bowel mass lesion pathologies diagnosed on device-assisted enteroscopic exams in our study. The slide to the left shows a well-differentiated NET. The slide to the right shows a hamartoma. NET: neuroendocrine tumor. |
Post-DAE lesion management
Thirty-seven point six percent of patients in this study were referred for surgical resection after diagnostic DAE. No further management was recommended for 26.1% of patients because the lesion was benign or completely resected endoscopically. Twenty-two point five percent of patients were advised to undergo endoscopic surveillance. Only 6.9% of patients were referred to medical oncology. The most common DAE interventions were tattooing (66.1%) followed by biopsy (61.1%) and endoscopic resection (38.4%). Among 218 patients there were only two reported DAE-related complications (0.92%), one perforation and one case of post-procedure bleeding. Both complications occurred during removal of large hamartomas in patients with PJS. One perforation occurred when a 1-cm ileal hamartoma on a thin stalk was removed with a hot snare without first utilizing a saline lift. The second complication was post-polypectomy bleeding after removal of several large proximal hamartomas in another patient. At the time of our retrospective chart review, 83% of patients included in this study were still alive (Tables 6, 7).
 Click to view | Table 6. Post-DAE Lesion Management |
 Click to view | Table 7. DAE Intervention |
| Discussion | ▴Top |
Small bowel neoplasm is rare, comprising only 3-6% of all GI tumors, often has a nonspecific presentation and is generally difficult to diagnose [3, 13, 14]. The advent and clinical adoption of VCE and DAE have dramatically improved our ability to detect, diagnose, and ultimately manage both benign and malignant SBMLs [3, 5, 8, 11, 15]. Owing to the fact that VCE and DAE now allow for evaluation of the entire small bowel, the incidence of malignant small bowel tumors has increased from 1-2% up to 4-9% [15-17]. There have been several recent studies highlighting the significant role of DAE, particularly DBE, plays in the diagnosis and management of SBMLs with decisive management only being possible after the lesion is reached and classified with DAE [15, 16, 18-20].
SBMLs typically present with nonspecific symptoms and most commonly arise in patients without polyposis or a family history of GI malignancy and diligence is needed when evaluating patients with persistent anemia or other insidious symptoms. We recommend using VCE as the best initial step when small bowel pathology is suspected. Though it can sometimes be challenging to distinguish true lesions, particularly when they are submucosal, from folds temporarily augmented by peristalsis or innocent bulges from extrinsic compression from other organs or loops of adjacent small bowel, scoring systems have been developed to assist in making this important distinction [3, 4, 21, 22]. VCE is better at identifying SBMLs than cross-sectional imaging and if a SBML is found, it can help guide the optimal direction of approach for subsequent DAE. Since most SBMLs are polypoid or submucosal, concern for capsule retention should not steer providers away from VCE, even when the suspicion for a SBML is high. Although DAE, especially DBE, remains a limited resource that is often only available in select academic centers, if a SBML is detected on VCE or cross-sectional imaging, we suggest trying to reach the lesion for direct assessment with DAE. To this end, any one of the three DAE modalities may be used for the direct visualization and assessment of the lesion in question. Our recent large, multi-center report published in 2021 revealed a high diagnostic yield (76.3%) and very low complication rate with no significant difference across all three DAE modalities [8]. Other studies have also shown no clinically significant variances when the different DAE modalities were compared head to head, though DBE has routinely been shown to achieve deeper depths of insertion and so more distal lesions may require this to be accessed [8, 21-26]. If the lesion is mucosal, DAE can potentially obviate the need for surgery by enabling definitive endoscopic resection or biopsy to confirm benign histology. If the lesion is submucosal or appears malignant, DAE can help guide operative management by providing a histologic diagnosis through biopsy and marking its location with a tattoo prior to subsequent surgical resection. If DAE is unavailable or VCE findings suggest that definitive endoscopic management will not be possible and there is an experienced surgeon willing to partner in the patient’s management, exploratory laparoscopy/laparotomy with intraoperative enteroscopy is still an appropriate alternative for managing these lesions, particularly if there is the potential for local metastases.
We acknowledge the limitations of this study. It was retrospective and involved three referral centers with no prospective protocol on how and when to utilize DAE in the management of these patients. Histology of biopsies and resected specimens were read by different pathologists across the different institutions and not centrally re-interpreted. Many of the patients were referred to our institutions specifically for DAE and so their antecedent course was not always clear. And because our cohort was drawn entirely from patients diagnosed by DAE, we did not analyze SBML that fell within reach of conventional upper or lower endoscopy.
Conclusions
In this present study we present a large cohort of patients with SBMLs diagnosed and managed with DAE across three tertiary referral centers. The vast majority of lesions (78.4%) were sporadic and hamartomas and NETs were the most common types of lesions found. Lipomas, inflammatory polyps, and hyperplastic polyps were all more common than primary adenocarcinoma of the small bowel which remains very rare. SBMLs most typically present in the sixth decade of life with anemia or GI bleeding and there can be a long lapse between symptom onset and diagnosis. Most patients undergo precursor evaluation and among the modalities used, VCE was superior to cross-sectional imaging at identifying SBMLs. If SBMLs are detected, DAE can enable definitive endoscopic management or important preoperative guidance.
Acknowledgments
The authors would like to acknowledge the Duke University Medical Center Department of Pathology for providing representative histology slides to be included in this publication.
Financial Disclosure
David R. Cave has received research funding from Olympus and Medtronic; the funding source had no role in the design, practice or analysis of this study. Otherwise, the authors have no financial or professional conflicts of interest.
Conflicts of Interest
The authors have no conflict of interest to declare.
Informed Consent
This study was designed and conducted in accordance with the IRB and consisted of retrospective chart review. The information in the manuscript is fully confidential and does not contain any individual patient identifiers. Given the nature of this study being large retrospective chart review, individual informed consent from patients was not required.
Author Contributions
MGN and DW thought of, designed, and coordinated this multi-center study. MGN, CD, LB, MH, DR, DC, and DW were responsible for chart review and data collection. MGN and DW wrote the manuscript and all subsequent revisions. AP and DN tabulated the raw data and performed the statistical analysis. DC, DR, AP, DN, and DW proofread the manuscript and all subsequent revisions. MGN created all tables and figures for publication.
Data Availability
The authors declare that data supporting the findings of this study are available within the article. Furthermore, the raw data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- de Latour RA, Kilaru SM, Gross SA. Management of small bowel polyps: A literature review. Best Pract Res Clin Gastroenterol. 2017;31(4):401-408.
doi pubmed - Williamson JM, Williamson RC. Small bowel tumors: pathology and management. J Med Assoc Thai. 2014;97(1):126-137.
- Min M, Noujaim MG, Green J, Schlieve CR, Vaze A, Cahan MA, Cave DR. Role of mucosal protrusion angle in discriminating between true and false masses of the small bowel on video capsule endoscopy. J Clin Med. 2019;8(4):418.
doi pubmed - Riccioni ME, Cianci R, Urgesi R, Bizzotto A, Spada C, Rizzo G, Coco C, et al. Advance in diagnosis and treatment of small bowel tumors: a single-center report. Surg Endosc. 2012;26(2):438-441.
doi pubmed - Noujaim MG, Green J, Min M, Schlieve CR, Patel K, Cahan M, Cave D. Carcinoids and capsules: a case series highlighting the utility of capsule endoscopy in patients with small bowel carcinoids. Gastroenterology Res. 2017;10(6):347-351.
doi pubmed - Cobrin GM, Pittman RH, Lewis BS. Increased diagnostic yield of small bowel tumors with capsule endoscopy. Cancer. 2006;107(1):22-27.
doi pubmed - Urgesi R, Riccioni ME, Bizzotto A, Cianci R, Spada C, Pelecca G, Ricci R, et al. Increased diagnostic yield of small bowel tumors with PillCam: the role of capsule endoscopy in the diagnosis and treatment of gastrointestinal stromal tumors (GISTs). Italian single-center experience. Tumori. 2012;98(3):357-363.
doi pubmed - Noujaim MG, Parish A, Raines D, Gross SA, Cave D, Vance I, Beyer D, et al. Use, yield, and risk of device-assisted enteroscopy in the United States: results from a large retrospective multicenter cohort. J Clin Gastroenterol. 2021;55(9):792-797.
doi pubmed - Moglia A, Menciassi A, Dario P, Cuschieri A. Clinical update: endoscopy for small-bowel tumours. Lancet. 2007;370(9582):114-116.
doi pubmed - Honda W, Ohmiya N, Hirooka Y, Nakamura M, Miyahara R, Ohno E, Kawashima H, et al. Enteroscopic and radiologic diagnoses, treatment, and prognoses of small-bowel tumors. Gastrointest Endosc. 2012;76(2):344-354.
doi pubmed - Gross SA, Stark ME. Initial experience with double-balloon enteroscopy at a U.S. center. Gastrointest Endosc. 2008;67(6):890-897.
doi pubmed - Yamamoto H, Sekine Y, Sato Y, Higashizawa T, Miyata T, Iino S, Ido K, et al. Total enteroscopy with a nonsurgical steerable double-balloon method. Gastrointest Endosc. 2001;53(2):216-220.
doi pubmed - Paski SC, Semrad CE. Small bowel tumors. Gastrointest Endosc Clin N Am. 2009;19(3):461-479.
doi pubmed - Almeida N, Figueiredo P, Lopes S, Gouveia H, Leitao MC. Double-balloon enteroscopy and small bowel tumors: a South-European single-center experience. Dig Dis Sci. 2009;54(7):1520-1524.
doi pubmed - Nishimura N, Mizuno M, Shimodate Y, Doi A, Mouri H, Matsueda K, Yamamoto H. The role of double-balloon enteroscopy in the diagnosis and surgical treatment of metastatic small bowel tumors. Intern Med. 2018;57(9):1209-1212.
doi pubmed - Cangemi DJ, Patel MK, Gomez V, Cangemi JR, Stark ME, Lukens FJ. Small bowel tumors discovered during double-balloon enteroscopy: analysis of a large prospectively collected single-center database. J Clin Gastroenterol. 2013;47(9):769-772.
doi pubmed - Mitsui K, Tanaka S, Yamamoto H, Kobayashi T, Ehara A, Yano T, Goto H, et al. Role of double-balloon endoscopy in the diagnosis of small-bowel tumors: the first Japanese multicenter study. Gastrointest Endosc. 2009;70(3):498-504.
doi pubmed - Chen WG, Shan GD, Zhang H, Li L, Yue M, Xiang Z, Cheng Y, et al. Double-balloon enteroscopy in small bowel tumors: a Chinese single-center study. World J Gastroenterol. 2013;19(23):3665-3671.
doi pubmed - Robles EP, Delgado PE, Conesa PB, Andres BM, Guggiana MF, Mateos EA, Caballero MF, et al. Role of double-balloon enteroscopy in malignant small bowel tumors. World J Gastrointest Endosc. 2015;7(6):652-658.
doi pubmed - He Q, Bai Y, Zhi FC, Gong W, Gu HX, Xu ZM, Cai JQ, et al. Double-balloon enteroscopy for mesenchymal tumors of small bowel: nine years' experience. World J Gastroenterol. 2013;19(11):1820-1826.
doi pubmed - Lipka S, Rabbanifard R, Kumar A, Brady P. Single versus double balloon enteroscopy for small bowel diagnostics: a systematic review and meta-analysis. J Clin Gastroenterol. 2015;49(3):177-184.
doi pubmed - Tang L, Huang LY, Cui J, Wu CR. Effect of double-balloon enteroscopy on diagnosis and treatment of small-bowel diseases. Chin Med J (Engl). 2018;131(11):1321-1326.
doi pubmed - Rahmi G, Samaha E, Vahedi K, Ponchon T, Fumex F, Filoche B, Gay G, et al. Multicenter comparison of double-balloon enteroscopy and spiral enteroscopy. J Gastroenterol Hepatol. 2013;28(6):992-998.
doi pubmed - Efthymiou M, Desmond PV, Brown G, La Nauze R, Kaffes A, Chua TJ, Taylor AC. SINGLE-01: a randomized, controlled trial comparing the efficacy and depth of insertion of single- and double-balloon enteroscopy by using a novel method to determine insertion depth. Gastrointest Endosc. 2012;76(5):972-980.
doi pubmed - May A, Farber M, Aschmoneit I, Pohl J, Manner H, Lotterer E, Moschler O, et al. Prospective multicenter trial comparing push-and-pull enteroscopy with the single- and double-balloon techniques in patients with small-bowel disorders. Am J Gastroenterol. 2010;105(3):575-581.
doi pubmed - Takano N, Yamada A, Watabe H, Togo G, Yamaji Y, Yoshida H, Kawabe T, et al. Single-balloon versus double-balloon endoscopy for achieving total enteroscopy: a randomized, controlled trial. Gastrointest Endosc. 2011;73(4):734-739.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


