| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Review
Volume 15, Number 6, December 2022, pages 297-307
An Open-Access, Interactive Decision-Support Tool to Facilitate Guideline-Driven Care for Hepatocellular Carcinoma
Robert J. Wonga, k, Channa Jayasekerab, Patricia Jonesc, Fasiha Kanwald, Amit G. Singale, Aijaz Ahmedf, Robert Taglientig, Zobair Younossih, Laura Kuliki, Neil Mehtaj
aVeterans Affairs Palo Alto Healthcare System, Stanford University School of Medicine, Palo Alto, CA, USA
bMayo Clinic, Phoenix, AZ, USA
cUniversity of Miami, Miami, FL, USA
dMichael E. DeBakey Veterans Affairs Medical Center and Baylor College of Medicine, Houston, TX, USA
eUniversity of Texas Southwestern Medical Center, Dallas, TX, USA
fStanford University School of Medicine, Stanford, CA, USA
gChronic Liver Disease Foundation, Clark, NJ, USA
hInova Fairfax Hospital, Falls Church, VA, USA
iNorthwestern University, Chicago, IL, USA
jUniversity of California, San Francisco, CA, USA
kCorresponding Author: Robert J. Wong, Veterans Affairs Palo Alto Healthcare System, Stanford University School of Medicine, Palo Alto, CA 94304, USA
Manuscript submitted September 28, 2022, accepted November 7, 2022, published online December 1, 2022
Short title: A Digital Tool for Hepatocellular Carcinoma
doi: https://doi.org/10.14740/gr1573
- Abstract
- Introduction
- The Current State of HCC Surveillance in the USA
- HCC Surveillance Disparities
- Efforts to Improve HCC Surveillance
- A Digital, Decision-Support Tool for Improving the HCC Cascade of Care
- Conclusions and Future Directions
- References
| Abstract | ▴Top |
Hepatocellular carcinoma (HCC) is increasing in incidence and is a leading cause of cancer-related mortality worldwide. Adherence to HCC surveillance guidelines and appropriate treatment triage of liver lesions may improve receipt of curative-intent treatment and improved survival. Late-stage HCC diagnosis reflects sub-optimal implementation of effective HCC surveillance, whereas inappropriate treatment triage or linkage to care accounts for the non-receipt of curative-intent in close to half of early-stage HCC in the USA. A free, open-access decision-support tool for liver lesions that incorporates current guideline recommendations in a user-friendly interface could improve appropriate and timely triage of patients to appropriate care. This review provides a summary of gaps and disparities in linkage to HCC care and introduces a free, internet-based, interactive decision-support tool for managing liver lesions. This tool has been developed by the HCC Steering Committee of the Chronic Liver Disease Foundation and is targeted toward clinicians across specialties who may encounter liver lesions during routine care or as part of dedicated HCC surveillance.
Keywords: Digital decision-support tool; Hepatocellular carcinoma; Surveillance; Barcelona Clinic Liver Cancer staging system; Cascade of care
| Introduction | ▴Top |
Hepatocellular carcinoma (HCC) is the fourth most common cause of cancer-related mortality worldwide [1, 2]. Between 1990 and 2015, HCC incidence increased by 75% worldwide [3]. Globally, HCC results in over 800,000 annual deaths [4]; in the USA, it is the fastest-rising cause of cancer mortality [5, 6]. A recent study using data from the Global Burden of Disease Study reported that liver cancer deaths increased by 25% from 2010 to 2019, which was likely driven primarily by non-alcoholic steatohepatitis (NASH) and alcohol-related liver disease, the two etiologies with the most rapid increase in age-standardized death rates during this time period [7]. Cirrhosis is the primary risk factor for HCC, with one-third of patients with cirrhosis ultimately developing HCC [3, 8]. Non-alcoholic fatty liver disease (NAFLD) has become the leading cause of chronic liver disease (CLD) in most regions of the world, including the USA [9, 10], where it is the fastest-growing etiology among patients with HCC undergoing liver transplantation (LT) [9, 11]. In addition to disease-specific factors affecting HCC risk, data also demonstrate that disparities in access to care, social support, and lifestyle also contribute to differences in long-term HCC risk and outcomes [9, 12].
While HCC is associated with high morbidity and mortality [3, 11], early detection improves options for potentially curative treatment, which is associated with significantly improved 5-year survival. Conversely, patients diagnosed with advanced stages of HCC survive a median of 1 year [13] and up to 2 years with systemic therapy. Effective implementation of HCC screening and surveillance in patients with cirrhosis is associated with improved early-stage diagnosis of HCC, while sub-optimal HCC surveillance has led to a high proportion of HCC being detected at advanced stages [8]. In addition to sub-optimal HCC surveillance, other gaps along the HCC cascade of care, including delays in accurate HCC diagnosis and accurate tumor staging, as well as timely linkage to appropriate HCC-directed therapies, contribute to poor patient outcomes [10, 11]. The Chronic Liver Disease Foundation (CLDF), a nonprofit educational organization dedicated to increasing awareness of the impact of CLD in the USA, assembled a group of HCC experts, who are also members of the CLDF, to form the HCC Steering Committee. Recognizing unmet needs in the HCC cascade of care, a small working group containing Steering Committee members was assembled and tasked with creating a practical, point-of-care tool to assist healthcare professionals in the surveillance, diagnosis, staging, and treatment of patients with HCC. This review seeks to increase awareness of the existing gaps in the HCC cascade of care by reviewing data on surveillance rates, disparities associated with HCC surveillance, and targeted efforts to improve HCC surveillance. It also presents a digital, decision-support tool for HCC, which incorporates guideline-based recommendations on HCC surveillance and staging systems. The terminology used throughout this review is in accordance with that followed by the American College of Radiology Liver Imaging Reporting and Data System (LI-RADS) (Fig. 1) [14].
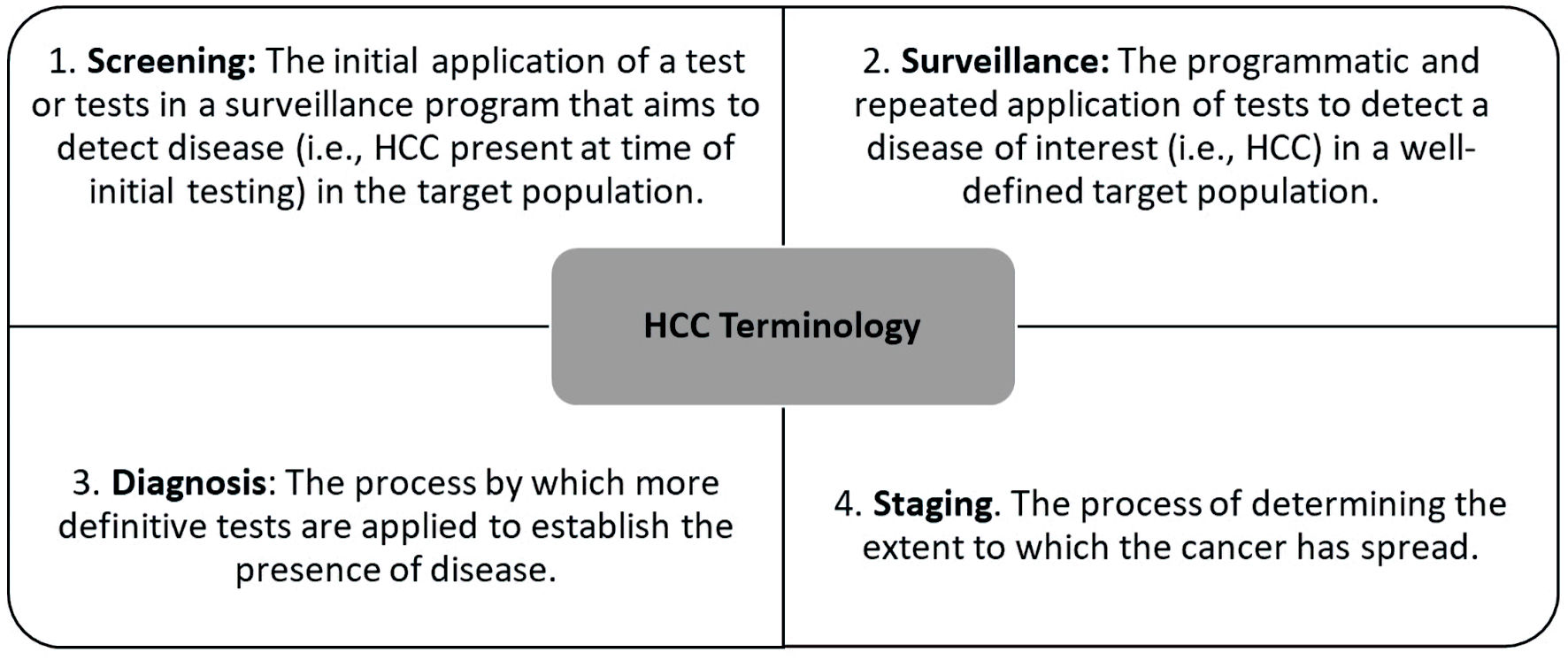 Click for large image | Figure 1. HCC terminology [14]. HCC: hepatocellular carcinoma. |
| The Current State of HCC Surveillance in the USA | ▴Top |
Cancer surveillance programs aim to detect tumors at an early stage when curative options are feasible [6, 15]. Professional societies, including the National Comprehensive Cancer Network [16], European Association for the Study of the Liver (EASL) [17], and American Association for Study of Liver Diseases (AASLD) [18], recommend HCC surveillance every 6 months with abdominal imaging with or without alpha-fetoprotein (AFP) in patients with cirrhosis. However, existing data illustrate sub-optimal HCC surveillance, which translates into poor HCC outcomes. For example, the Hepatitis C Antiviral Long-Term Treatment against Cirrhosis Trial (HALT-C) prospectively collected HCC surveillance data on a cohort of 1,005 patients over a mean follow-up of 6.1 years and found that 31.1% of the patients did not receive consistent surveillance [19]. Another study using data from the Surveillance, Epidemiology, and End Results (SEER) Program-Medicare database, which included 13,714 patients diagnosed with HCC from 2003 through 2013, observed that over 51% of patients with cirrhosis did not receive any screening in the 3 years before an HCC diagnosis and only 6.8% of patients underwent consistent annual screening. After correction for lead- and length-time biases, it was found that a higher proportion of patients with consistent (23%; 95% confidence interval (CI), 21-25%) and inconsistent screening (19%; 95% CI, 19-20%) survived for 3 years, compared to patients without screening (13%; 95% CI, 12-14%) [20]. Most recently, Wolf et al performed a systematic review and meta-analysis of studies published from 2010 through 2018. A total of 29 studies, inclusive of 118,799 patients, met the inclusion criteria. The authors observed that the pooled estimate for HCC surveillance was 24.0% (95% CI, 18.4-30.1%). Furthermore, patients with alcohol-associated or NASH-related cirrhosis and those not followed in subspecialty gastroenterology clinics had the lowest surveillance rates [21].
Data in Table 1 [22-27] support the benefits of early HCC surveillance, which includes improved early-stage tumor detection, curative treatment rates, and improved long-term survival [20, 22-26, 28]. Because there are no data from randomized controlled trials in patients with cirrhosis, the overall value of HCC screening in patients with cirrhosis has been questioned. Singal et al performed a systematic literature review from January 2014 through July 2020 for studies reporting early-stage HCC detection, curative treatment receipt, or overall survival, stratified by HCC surveillance status, among patients with cirrhosis. They identified 59 studies, including 145,396 patients with HCC that was detected by surveillance in 41,052 (28.2%) cases. HCC surveillance was associated with improved early-stage detection (relative risk (RR) 1.86, 95% CI, 1.73 - 1.98; I2 = 82%), curative treatment receipt (RR 1.83, 95% CI, 1.69 - 1.97; I2 = 75%), and overall survival (hazard ratio 0.67, 95% CI, 0.61 - 0.72; I2 = 78%) after adjusting for lead-time bias. Although there was notable heterogeneity in all pooled estimates and little data quantifying potential screening-related harms, the authors concluded that screening is of high value in patients with cirrhosis [29].
 Click to view | Table 1. Data Demonstrating the Benefits of Early HCC Surveillance and/or Screening |
| HCC Surveillance Disparities | ▴Top |
In order to improve HCC surveillance rates and meet individualized patient needs, it is important to understand that surveillance may be impacted by certain factors, which will be explored in this section.
Patient barriers
Sub-optimal HCC surveillance is affected by multiple factors. An important and perhaps underrecognized factor that may contribute to lower rates of HCC surveillance is patient distress and/or patient stigma. Surveillance may result in depression or anxiety from the screening process; financial burdens and/or physical harms due to false-positives or inconclusive results; and adverse effects from overdiagnoses [8]. Patient-perceived barriers also negatively impact surveillance rates; in a patient survey, perceived barriers included costs (28.9%), difficulty scheduling (24.1%), and transportation (17.8%). In addition, although these patients had high levels of knowledge about HCC, there was a misconception that surveillance was unnecessary when physical examination and laboratory results were normal [30].
Racial and ethnic disparities
Significant racial and ethnic disparities in HCC surveillance have been reported. For example, existing studies have identified significantly lower rates of HCC surveillance in Black patients when compared with other race/ethnic groups [31-33]. These are concerning findings, given that there are significant racial and ethnic disparities in HCC prognosis in the USA, especially in Black patients, as detailed in Table 2 [33-36]. Black patients demonstrate higher HCC incidence, more advanced tumor stage at diagnosis, lower rates of surgical resection, and higher mortality rates as compared to non-Blacks. These disparities may be attributed to increased risk factors as well as disparities in timely access to healthcare resources and differences in barriers, medical mistrust, and health literacy [33, 37]. Racial and ethnic disparities may also be impacted by socioeconomic status, as the two are often closely intertwined [38]. As detailed in Table 2 [33-36], one study concluded that Black patients with hepatitis C virus (HCV) exposure develop HCC at earlier stages of liver disease than members of other racial groups [34]. Interventions are needed to better understand and reduce disparities in early HCC detection to improve HCC prognosis [31].
 Click to view | Table 2. HCC Disproportionally Affects Racial and Ethnic Subgroups |
Impact of geography on HCC disparities
Disparities in HCC surveillance and the HCC cascade of care may also be affected by where a patient lives, as those in more rural areas may not have timely access to specialty care or may have difficulty accessing services for HCC surveillance. Wong et al retrospectively evaluated the 2004 - 2017 SEER registry and found that, among men and women, overall HCC incidence was highest in large metropolitan regions and decreased with less populated/more rural regions; however, less populated/more rural regions demonstrated the greatest increase in HCC incidence over time. Investigators also observed higher HCC incidence and higher average annual percent change in HCC incidence among lower income households and higher HCC incidence in lower income groups. These disparities were attributed to differences in risk factors, health-related behaviors, and barriers in access to healthcare services inherent among underserved, rural, immigrant-rich communities [39]. In a separate analysis of the same database, trends in HCC incidence by rural-urban geography and household income and how these surrogate markers of socioeconomic status affect HCC tumor stage at diagnosis and overall survival were evaluated. Investigators found that patients from rural regions and lower-income households had a more advanced tumor stage at diagnosis and significantly higher HCC mortality, likely reflecting suboptimal access to consistent high-quality liver disease care, including HCC surveillance [40]. While the rural-urban disparities in HCC outcomes are complex and likely reflect multiple factors, limited access to specialty care services is one important modifiable factor that could be addressed. An easy to use, digital, decision support tool has the potential to help providers in rural areas with limited access to specialty care to better identify high-risk patients that need HCC surveillance, what surveillance modalities to use, and how frequently to image, and provide guidance on interpretation and follow-up of liver lesions that are identified.
Inadequate linkage to care
In a retrospective study conducted by Marquardt et al [41], the most common barrier to HCC screening was lack of routine outpatient care before HCC presentation, with nearly two-thirds of patients failing to receive regular care from a primary care provider (PCP) or gastroenterologist. In a separate Veterans Health Administration study that analyzed 26,577 patients with cirrhosis, the strongest predictor that increased the percentage of time up-to-date with HCC surveillance was the number of visits to a specialist (gastroenterologist/hepatologist and/or infectious disease specialist) in the first year after cirrhosis was diagnosed [42]. In other studies, linkage to specialist care was strongly associated with increased surveillance rates [43-47], and this was attributed to the specialist having the ability to focus solely on the liver during visits [47].
| Efforts to Improve HCC Surveillance | ▴Top |
As demonstrated throughout this review, low rates of HCC surveillance are multi-factorial and likely reflect complex interactions with patient, provider, and overall health system factors [42].
Improving HCC surveillance and diagnosis by automation
In this digital age, automation is revolutionizing healthcare by making processes more efficient and effective, thereby improving patient outcomes [48]. Automation empowers practitioners to offer better services to patients, and this has been demonstrated with HCC surveillance. Patients with cirrhosis (n = 355) were prospectively enrolled into a chronic disease management program that integrates nursing-based protocols with automatic reminders when patients are due for surveillance. Patients enrolled in this program between March 2010 and April 2011 were compared to a prior cohort in 2008 - 2009. Automatic reminders of surveillance status demonstrated significant increases in the rate of HCC surveillance among patients with cirrhosis; the results indicated that 331 (93%) had imaging performed for HCC surveillance, compared to 119/160 (74%) patients in the previous cohort (P < 0.001). The most common reasons for failure to undergo surveillance were patients’ lack of insurance and lack of follow-up on studies ordered at outside institutions [49].
Another study examined the efficiency and validity of using automated data to identify HCC cases. Investigators used a cohort of 1,138 veterans with International Classification of Diseases, ninth Revision (ICD-9) codes for HCC during 2005 - 2010 to validate HCC codes and evaluate whether natural language processing by the Automated Retrieval Console (ARC) for document classification improves HCC identification. The HCC ICD-9 code algorithm had a positive predictive value (PPV) of 0.67, sensitivity of 0.95, and specificity of 0.93. For a random subset of 619 patients, 471 pathology reports were identified for 323 patients and 943 radiology reports were identified for 557 patients. The pathology ARC algorithm had a PPV of 0.96, sensitivity of 0.96, and specificity of 0.97. The radiology ARC algorithm had a PPV of 0.75, sensitivity of 0.94, and specificity of 0.68. These results indicate that HCC case identification improved with utilization of automated data in comparison to a combined approach of ICD-9 codes and natural language processing of pathology and radiology reports [50].
Increasing PCP awareness of HCC surveillance
HCC surveillance is improved when specialists are involved in the care of patients with cirrhosis. On the other hand, in regions and/or systems where specialists are not readily available (e.g., rural areas) [47], PCPs may have the opportunity to take the lead on implementing effective HCC surveillance. However, existing survey-based studies focused on PCPs indicate that lack of awareness of the HCC surveillance guidelines, sub-optimal knowledge regarding proper HCC surveillance, failure to recognize liver disease in patients that warrant HCC surveillance, and time constraints were observed to be important limiting factors that contributed to provider-specific barriers to timely HCC surveillance [51, 52]. On the contrary, PCPs felt that, if medical societies endorsed evidence-based guidelines for HCC surveillance, they would be motivated to comply [52].
| A Digital, Decision-Support Tool for Improving the HCC Cascade of Care | ▴Top |
Efforts are needed to improve HCC surveillance. For HCC surveillance to reduce mortality in clinical practice, it must be effectively implemented [52]. Data suggest that decision-support tools for HCC can help standardize the diagnosis, staging, linkage-to-care, and treatment pathways, thereby improving overall patient care and outcomes [4]. The CLDF HCC committee developed a practical, interactive, digital decision-support tool to improve the HCC cascade of care. This web-based tool is designed to assist healthcare professionals in screening and surveillance, tumor staging, and treatment of patients with HCC, based on the following resources: 1) The most recently updated guidance on HCC management from the AASLD and EASL, which deliver data-supported approaches to the diagnosis, staging, and treatment of patients diagnosed with HCC [17, 18]; 2) The American College of Radiology LI-RADS criteria, which standardize terminology, technique, interpretation, reporting, and data collection of liver imaging in patients at risk for or with HCC [53]; 3) Incorporates most commonly used HCC staging systems, including the Barcelona Clinic Liver Cancer (BCLC) staging system [54] and the United Network for Organ Sharing-Down Staging (UNOS-DS) criteria [55].
We aim for this to be a live decision-support tool that will be updated as HCC management guidelines evolve and will also serve as a platform to build out future CLDF HCC initiatives to improve the care of patients with CLDs and HCC.
The HCC screening and surveillance algorithm
The HCC screening and surveillance algorithm is depicted in Figure 2 [56]. According to the AASLD guidance, the decision to enter a patient into a surveillance program for HCC is risk-stratified. The AASLD recommends surveillance of adults with cirrhosis as well as certain individuals with chronic hepatitis B virus (HBV) who are determined to be at high risk of HCC [18]. Thus, the first and one of the most important steps in the HCC cascade of care is accurate identification of which patients are eligible for routine HCC surveillance (Fig. 2) [56]. Among patients with cirrhosis or high-risk non-cirrhotic chronic HBV (Asian women over age of 50 years, Asian or Black/African American men over age of 40 years, family history of HCC, co-infection with hepatitis D virus (HDV)), the AASLD recommends surveillance using ultrasound, with or without AFP, every 6 months [18]. If a liver lesion is not detected on ultrasound or measures < 1 cm, or AFP is < 20 ng/mL, continued screening for HCC with abdominal ultrasound examination with or without AFP every 6 months is recommended [18]. If a liver lesion is detected and measures ≥ 1 cm or AFP > 20 ng/mL, further evaluation is required. The AASLD recommends either multiphase computed tomography (CT) or magnetic resonance imaging (MRI), which have similar performance characteristics for initial diagnostic testing. It is important to note that the accuracy of ultrasound results relies on high-quality examinations with adequate visualization and assessment of lesions seen. If ultrasound visualization quality is sub-optimal, subsequent imaging with multi-phase CT or MRI is recommended.
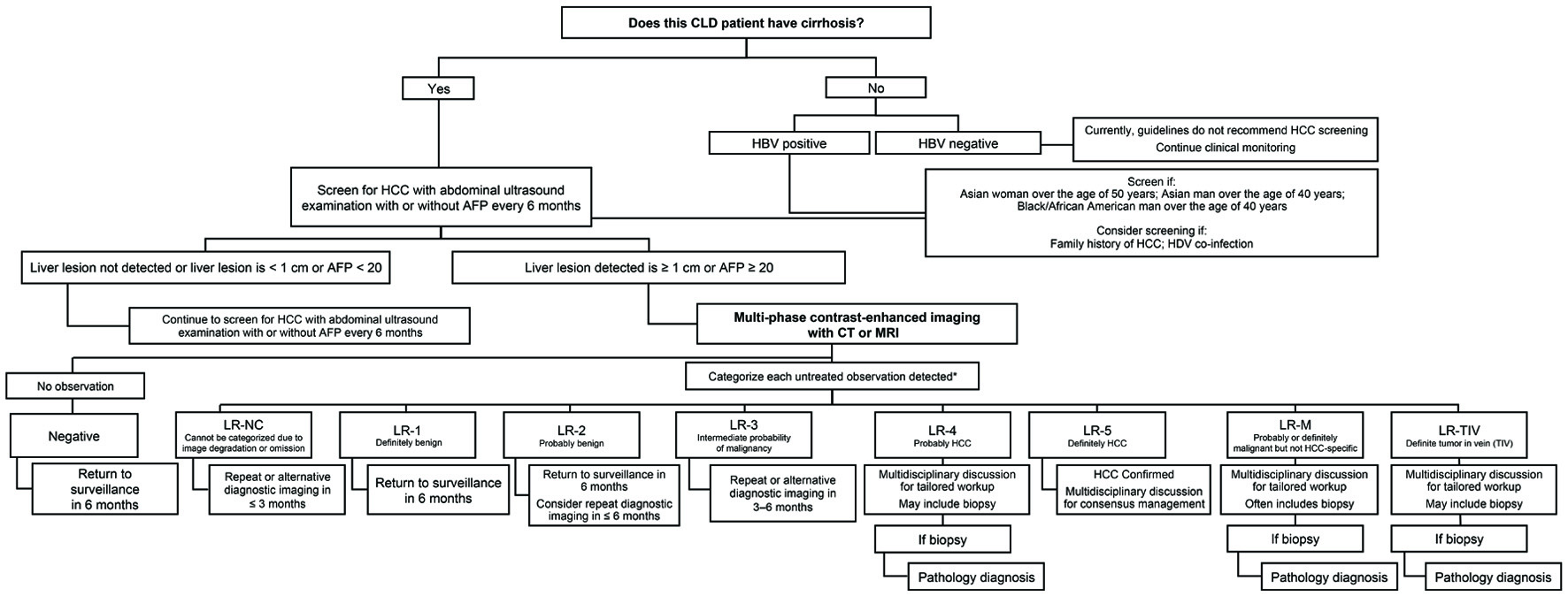 Click for large image | Figure 2. HCC screening and surveillance algorithm. *For more information on “The Liver Imaging Reporting and Data System (LI-RADS),” visit [56]. AFP: alpha fetoprotein; CLD: chronic liver disease; CT: computed tomography; HBV: hepatitis B virus; HCC: hepatocellular carcinoma; HDV: hepatitis D virus; LR: Liver Imaging Reporting and Data System score; M: malignancy; MRI: magnetic resonance imaging; NC: not categorizable; TIV: tumor in vein. |
The HCC staging algorithm
Once HCC diagnosis is confirmed, accurate tumor staging is needed to help guide the most effective evidenced-based therapy (Fig. 3) [54]. This staging algorithm was adapted from the BCLC staging system and uses variables related to tumor stage, liver functional status/severity of hepatic decompensation, performance status, and cancer-related symptoms, and links each stage with guideline-recommended treatment options [54]. The BCLC staging system incorporates the Eastern Cooperative Oncology Group (ECOG) scale (Table 3) [57] to assess for patient functional status [57].
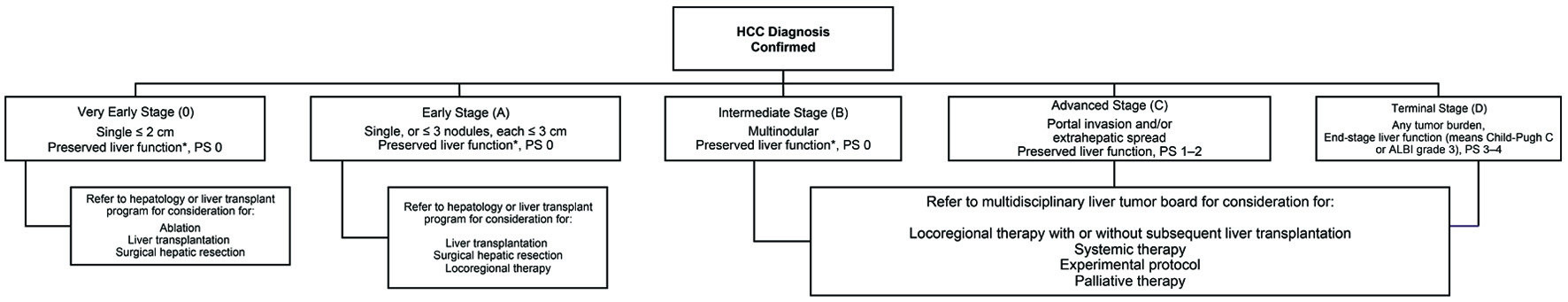 Click for large image | Figure 3. HCC staging algorithm [54]. HCC: hepatocellular carcinoma; ALBI: albumin-bilirubin; PS: ECOG performance status. |
 Click to view | Table 3. ECOG Performance Scale [57] |
Implementing effective screening is important to diagnose patients with HCC at an earlier stage, which improves eligibility for potentially curative treatment options, such as surgical resection and LT. LT is thought to be the better oncologic option, as it replaces the diseased liver and thus restores normal hepatic function, but it is limited by organ shortages. The Milan criteria [58] remains an important factor in identifying patients with HCC with favorable characteristics associated with good outcomes following LT. However, data have demonstrated that additional factors, beyond tumor size and number, play a role in post-LT outcomes, and even among patients that have HCC beyond Milan criteria at diagnosis, LT is not completely eliminated as a treatment option. (i.e., UNOS downstaging for HCC beyond Milan criteria) [55, 59, 60]. Patients with initial tumor burden meeting UNOS-DS inclusion criteria who achieve successful down-staging to within Milan criteria are eligible to receive automatic approval for Model for End-Stage Liver Disease (MELD) exception for LT whereas patients initially beyond UNOS-DS criteria are considered on a case-by-case basis. Furthermore, additional refinements based on AFP cutoffs may further improve post-LT outcomes [61-63]. Therefore, this staging algorithm recommends considering these additional criteria (Table 4) [55, 58, 61] based on the BCLC stage when making decisions on next steps in certain patients.
 Click to view | Table 4. Liver Transplantation Criteria in Early to Intermediate Stage HCC Additional Staging Criteria to Consider in BCLC-Staged Patients |
| Conclusions and Future Directions | ▴Top |
These proposed algorithms, based on published literature and an iterative process that included discussions among expert clinicians, and updates to the algorithms, are currently posted on the CLDF website [64]. The algorithms will be updated on a regular basis (i.e., at least annually depending on the publication of relevant updates in the management of HCC) as appropriate and will be used as educational tools for health care providers. As discussed, the current digital, interactive, HCC decision-support tool aims to improve the cascade of care for patients at risk for HCC. The live and real-time version of the HCC tool will allow providers to seamlessly navigate through a decision-making algorithm that integrates HCC screening and surveillance with accurate tumor staging followed by linking tumor stage to recommended HCC-directed therapies based on the BCLC guidance. Future iterations of this interactive tool have enormous potential to link patients with HCC to specific providers that provide the recommended HCC-directed therapies in their respective regions. This next step may further address existing gaps in the HCC cascade of care and reduce HCC treatment disparities.
Acknowledgments
We would like to thank Rachel E. Bejarano, PharmD for providing medical writing assistance.
Financial Disclosure
This manuscript was supported by an unrestricted educational grant to the Chronic Liver Disease Foundation from AstraZeneca and Exelixis, Inc. The selection of the authors and the creation of this manuscript were done independently, and AstraZeneca and Exelixis, Inc. did not play a role.
Conflict of Interest
Robert J. Wong, MD, Consultant: Gilead Sciences; Research funding: Gilead Sciences (to my institution), Exact Sciences (to my institution). Patricia Jones, MD: Gilead Sciences (to my institution), NIH grants that pay some salary support: 1R01MD012565-03, 1U01DK130185-01, 1R01MD017063-01, 1K08CA255413-10A1; V Foundation provides some salary support. Fasiha Kanwal, MD is supported by National Cancer Institute (NCI U01 CA230999, and R01CA186566), Cancer Prevention & Research Institute of Texas grant (RP150587), VA Merit Grant (IIR 21-230-2) and is an investigator at the Veterans Administration Center for Innovations in Quality, Effectiveness and Safety (CIN 13-413), Michael E. DeBakey VA Medical Center, Houston, Texas; Research funding: Gilead Sciences (to my institution), Exact Sciences (to my institution). Amit Singal, MD, Consultant: Genentech, AstraZeneca, Bayer, Eisai, Exelixis, Glycotest, FujiFilm Medical Sciences, Exact Sciences, Roche, Freenome, GRAIL, TARGET RWE; Research funding: supported by NCI U01 CA271888, U01 CA230694, R01 CA212008, R01 CA222900, R01 MD012565, and R01 CA256977. Zobair Younossi, MD, Research funding and/or Consultant: Gilead Sciences, Intercept, BMS, Novo Nordisk, Viking, Terns, Siemens, Shionogi, AbbVie, Merck, and Novartis. Laura Kulik, MD, Advisory role: Exelixis; Consultant: Bayer, Eisai, Genetec, Hepion, Merck; Speaker: Eisai, Roche; Research funding: Glycotest, HCC Target. Neil Mehta, MD, Consultant: FujiFilm Medical Sciences, Exact Sciences; Research funding: Glycotest, TARGET RWE, FujiFilm Medical Sciences, Exact Sciences (all to my institution). Channa Jayasekera, MD, Aijaz Ahmed, MD, and Robert Taglienti have nothing to disclose.
Author Contributions
Robert J. Wong, MD, developed the HCC decision-support tool algorithm and other practical recommendations in the manuscript, co-led the group of expert hepatologists and drafted the proposed outline, identified the most recent updates in HCC data that pertain to the HCC screening and staging, drafted the manuscript, finalized the submitted draft, and revised the resubmitted draft and responded to journal reviewers. Channa Jayasekera, MD, Patricia Jones, MD, Fasiha Kanwal, MD, Amit G. Singal, MD, Zobair Younossi, MD, Laura Kulik, MD, and Neil Mehta, MD reviewed and provided feedback on the HCC decision-support tool algorithm and other practical recommendations in the manuscript, contributed to the most recent updates in HCC data that pertain to the HCC screening and staging, provided expert review and added content to the manuscript, and approved the final draft of the submitted manuscript. Aijaz Ahmed, MD, developed the HCC decision-support tool algorithm and other practical recommendations in the manuscript, co-led the group of expert hepatologists, identified the most recent updates in HCC data that pertain to the HCC screening and staging, drafted the manuscript, and approved the final draft of the submitted manuscript. Robert Taglienti led the programming of the online HCC decision-support tool algorithm with collaboration from the expert hepatologist panel, launched the live online support tool, and approved the final draft of the submitted manuscript.
Data Availability
The authors declare that data supporting what is stated in this paper are available within the article
Abbreviations
ALBI: albumin-bilirubin; AASLD: American Association for Study of Liver Diseases; AFP: alpha-fetoprotein; ARC: Automated Retrieval Console; BCLC: Barcelona Clinic Liver Cancer; CLD: chronic liver disease; CLDF: Chronic Liver Disease Foundation; CT: computed tomography; DS: down-staging; EASL: European Association for the Study of the Liver; ECOG: Eastern Cooperative Oncology Group; HALT-C: Hepatitis C Antiviral Long-Term Treatment against Cirrhosis Trial; HCC: hepatocellular carcinoma; HCV: hepatitis C virus; ICD-9: International Classification of Diseases, Ninth Revision; LI-RADS: American College of Radiology Liver Imaging Reporting and Data System; LRT: locoregional therapy; LT: liver transplantation; MRI: magnetic resonance imaging; MELD: Model for End-Stage Liver Disease; NAFLD: nonalcoholic fatty liver disease; NASH: non-alcoholic steatohepatitis; OS: overall survival; PCP: primary care provider; PS: ECOG performance status; SEER: Surveillance, Epidemiology, and End Results; TTD: total tumor diameter; UNOS: United Network of Organ Sharing; VA: Veterans’ Affairs
| References | ▴Top |
- Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450-1462.
doi - World Health Organization. Liver factsheet. Globocan. Available at: https://gco.iarc.fr/today/data/factsheets/cancers/11-Liver-fact-sheet.pdf.
- Sangiovanni A, Prati GM, Fasani P, Ronchi G, Romeo R, Manini M, Del Ninno E, et al. The natural history of compensated cirrhosis due to hepatitis C virus: A 17-year cohort study of 214 patients. Hepatology. 2006;43(6):1303-1310.
doi - Ferlay J, Ervik M, Lam F, et al. Global cancer observatory: cancer tomorrow. Lyon: International Agency for Research on Cancer. 2018. Available at: https://gco.iarc.fr/tomorrow. Accessed September 27, 2022.
- Liver Cancer. Available at: https://www.cdc.gov/cancer/liver/index.htm. Accessed June 16, 2022.
- Kulik L, El-Serag HB. Epidemiology and management of hepatocellular carcinoma. Gastroenterology. 2019;156(2):477-491.e471.
doi - Huang DQ, Singal AG, Kono Y, Tan DJH, El-Serag HB, Loomba R. Changing global epidemiology of liver cancer from 2010 to 2019: NASH is the fastest growing cause of liver cancer. Cell Metab. 2022;34(7):969-977.e962.
doi - Singal AG, Lampertico P, Nahon P. Epidemiology and surveillance for hepatocellular carcinoma: New trends. J Hepatol. 2020;72(2):250-261.
doi - Fassio E, Diaz S, Santa C, Reig ME, Martinez Artola Y, Alves de Mattos A, Miguez C, et al. Etiology of hepatocellular carcinoma in Latin America: a prospective, multicenter, international study. Ann Hepatol. 2010;9(1):63-69.
doi - Rao A, Rich NE, Marrero JA, Yopp AC, Singal AG. Diagnostic and therapeutic delays in patients with hepatocellular carcinoma. J Natl Compr Canc Netw. 2021;19(9):1063-1071.
doi - Singal AG, Lok AS, Feng Z, Kanwal F, Parikh ND. Conceptual model for the hepatocellular carcinoma screening continuum: current status and research agenda. Clin Gastroenterol Hepatol. 2022;20(1):9-18.
doi - McGlynn KA, London WT. Epidemiology and natural history of hepatocellular carcinoma. Best Pract Res Clin Gastroenterol. 2005;19(1):3-23.
doi - Llovet JM, Bustamante J, Castells A, Vilana R, Ayuso Mdel C, Sala M, Bru C, et al. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29(1):62-67.
doi - LI-RADS Manual, Chapter 2. Available at: https://www.acr.org/-/media/ACR/Files/Clinical-Resources/LIRADS/LI-RADS-2018-Manual-5Dec18.pdf. Accessed June 28, 2022.
- Prasad V, Lenzer J, Newman DH. Why cancer screening has never been shown to "save lives"—and what we can do about it. BMJ. 2016;352:h6080.
doi - National Comprehensive Cancer Network Liver Cancer. Available at: www.nccn.org. Accessed June 29, 2022.
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182-236.
- Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723-750.
doi - Singal AG, Nehra M, Adams-Huet B, Yopp AC, Tiro JA, Marrero JA, Lok AS, et al. Detection of hepatocellular carcinoma at advanced stages among patients in the HALT-C trial: where did surveillance fail? Am J Gastroenterol. 2013;108(3):425-432.
doi - Choi DT, Kum HC, Park S, Ohsfeldt RL, Shen Y, Parikh ND, Singal AG. Hepatocellular carcinoma screening is associated with increased survival of patients with cirrhosis. Clin Gastroenterol Hepatol. 2019;17(5):976-987.e974.
doi - Wolf E, Rich NE, Marrero JA, Parikh ND, Singal AG. Use of hepatocellular carcinoma surveillance in patients with cirrhosis: a systematic review and meta-analysis. Hepatology. 2021;73(2):713-725.
doi - Mittal S, Kanwal F, Ying J, Chung R, Sada YH, Temple S, Davila JA, et al. Effectiveness of surveillance for hepatocellular carcinoma in clinical practice: A United States cohort. J Hepatol. 2016;65(6):1148-1154.
doi - Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med. 2014;11(4):e1001624.
doi - van Meer S, de Man RA, Coenraad MJ, Sprengers D, van Nieuwkerk KM, Klumpen HJ, Jansen PL, et al. Surveillance for hepatocellular carcinoma is associated with increased survival: Results from a large cohort in the Netherlands. J Hepatol. 2015;63(5):1156-1163.
doi - Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130(7):417-422.
doi - Wu CY, Hsu YC, Ho HJ, Chen YJ, Lee TY, Lin JT. Association between ultrasonography screening and mortality in patients with hepatocellular carcinoma: a nationwide cohort study. Gut. 2016;65(4):693-701.
doi - Singal AG, Mittal S, Yerokun OA, Ahn C, Marrero JA, Yopp AC, Parikh ND, et al. Hepatocellular carcinoma screening associated with early tumor detection and improved survival among patients with cirrhosis in the US. Am J Med. 2017;130(9):1099-1106.e1091.
doi - Costentin CE, Layese R, Bourcier V, Cagnot C, Marcellin P, Guyader D, Pol S, et al. Compliance with hepatocellular carcinoma surveillance guidelines associated with increased lead-time adjusted survival of patients with compensated viral cirrhosis: a multi-center cohort study. Gastroenterology. 2018;155(2):431-442.e410.
doi - Singal AG, Zhang E, Narasimman M, Rich NE, Waljee AK, Hoshida Y, Yang JD, et al. HCC surveillance improves early detection, curative treatment receipt, and survival in patients with cirrhosis: A meta-analysis. J Hepatol. 2022;77(1):128-139.
doi - Singal AG, Tiro JA, Murphy CC, Blackwell JM, Kramer JR, Khan A, Liu Y, et al. Patient-reported barriers are associated with receipt of hepatocellular carcinoma surveillance in a multicenter cohort of patients with cirrhosis. Clin Gastroenterol Hepatol. 2021;19(5):987-995.e981.
doi - Rich NE, Carr C, Yopp AC, Marrero JA, Singal AG. Racial and ethnic disparities in survival among patients with hepatocellular carcinoma in the United States: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2022;20(2):e267-e288.
doi - Rich NE, Hester C, Odewole M, Murphy CC, Parikh ND, Marrero JA, Yopp AC, et al. Racial and ethnic differences in presentation and outcomes of hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2019;17(3):551-559.e551.
doi - Yu L, Sloane DA, Guo C, Howell CD. Risk factors for primary hepatocellular carcinoma in black and white Americans in 2000. Clin Gastroenterol Hepatol. 2006;4(3):355-360.
doi - Shaltiel T, Zheng S, Siderides C, Gleeson EM, Carr J, Pletcher ER, Cohen NA, et al. Hepatitis C-positive Black patients develop hepatocellular carcinoma at earlier stages of liver disease and present with a more aggressive phenotype. Cancer. 2021;127(9):1395-1406.
doi - Altekruse SF, Henley SJ, Cucinelli JE, McGlynn KA. Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. Am J Gastroenterol. 2014;109(4):542-553.
doi - Ha J, Yan M, Aguilar M, Bhuket T, Tana MM, Liu B, Gish RG, et al. Race/ethnicity-specific disparities in cancer incidence, burden of disease, and overall survival among patients with hepatocellular carcinoma in the United States. Cancer. 2016;122(16):2512-2523.
doi - Schoenberger H, Rich NE, Jones P, Yekkaluri S, Yopp A, Singal AG, Multi-Ethnic HCCCI. Racial and ethnic disparities in barriers to care in patients with hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2021.
doi - Wagle NS, Park S, Washburn D, Ohsfeldt RL, Rich NE, Singal AG, Kum HC. Racial, ethnic, and socioeconomic disparities in curative treatment receipt and survival in hepatocellular carcinoma. Hepatol Commun. 2022;6(5):1186-1197.
doi - Wong RJ, Saab S, Konyn P, Sundaram V, Khalili M. Rural-Urban Geographical Disparities in Hepatocellular Carcinoma Incidence Among US Adults, 2004-2017. Am J Gastroenterol. 2021;116(2):401-406.
doi - Wong RJ, Kim D, Ahmed A, Singal AK. Patients with hepatocellular carcinoma from more rural and lower-income households have more advanced tumor stage at diagnosis and significantly higher mortality. Cancer. 2020;127(1):45-55.
doi - Marquardt P, Liu PH, Immergluck J, Olivares J, Arroyo A, Rich NE, Parikh ND, et al. Hepatocellular carcinoma screening process failures in patients with cirrhosis. Hepatol Commun. 2021;5(9):1481-1489.
doi - Goldberg DS, Taddei TH, Serper M, Mehta R, Dieperink E, Aytaman A, Baytarian M, et al. Identifying barriers to hepatocellular carcinoma surveillance in a national sample of patients with cirrhosis. Hepatology. 2017;65(3):864-874.
doi - Patwardhan V, Paul S, Corey KE, Mazhar SM, Richter JM, Thiim M, Chung RT. Hepatocellular carcinoma screening rates vary by etiology of cirrhosis and involvement of gastrointestinal sub-specialists. Dig Dis Sci. 2011;56(11):3316-3322.
doi - Singal AG, Yopp AC, Gupta S, Skinner CS, Halm EA, Okolo E, Nehra M, et al. Failure rates in the hepatocellular carcinoma surveillance process. Cancer Prev Res (Phila). 2012;5(9):1124-1130.
doi - Singal AG, Li X, Tiro J, Kandunoori P, Adams-Huet B, Nehra MS, Yopp A. Racial, social, and clinical determinants of hepatocellular carcinoma surveillance. Am J Med. 2015;128(1):90.e91-97.
doi - El-Serag HB, Alsarraj A, Richardson P, Davila JA, Kramer JR, Durfee J, Kanwal F. Hepatocellular carcinoma screening practices in the Department of Veterans Affairs: findings from a national facility survey. Dig Dis Sci. 2013;58(11):3117-3126.
doi - Sherman M. Surveillance for hepatocellular carcinoma. Semin Oncol. 2001;28(5):450-459.
doi - Key Benefits of Automation in Healthcare. Available at: https://www.relatient.com/automation-in-healthcare. Accessed June 28, 2022.
- Aberra FB, Essenmacher M, Fisher N, Volk ML. Quality improvement measures lead to higher surveillance rates for hepatocellular carcinoma in patients with cirrhosis. Dig Dis Sci. 2013;58(4):1157-1160.
doi - Sada Y, Hou J, Richardson P, El-Serag H, Davila J. Validation of case finding algorithms for hepatocellular cancer from administrative data and electronic health records using natural language processing. Med Care. 2016;54(2):e9-14.
doi - Dalton-Fitzgerald E, Tiro J, Kandunoori P, Halm EA, Yopp A, Singal AG. Practice patterns and attitudes of primary care providers and barriers to surveillance of hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol. 2015;13(4):791-798.e791.
doi - McGowan CE, Edwards TP, Luong MU, Hayashi PH. Suboptimal surveillance for and knowledge of hepatocellular carcinoma among primary care providers. Clin Gastroenterol Hepatol. 2015;13(4):799-804.
doi - LI-RADS Manual, Chapter 1. Available at: https://www.acr.org/-/media/ACR/Files/Clinical-Resources/LIRADS/LI-RADS-2018-Manual-5Dec18.pdf. Accessed June 28, 2022.
- Reig M, Forner A, Rimola J, Ferrer-Fabrega J, Burrel M, Garcia-Criado A, Kelley RK, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76(3):681-693.
doi - Yao FY, Mehta N, Flemming J, Dodge J, Hameed B, Fix O, Hirose R, et al. Downstaging of hepatocellular cancer before liver transplant: long-term outcome compared to tumors within Milan criteria. Hepatology. 2015;61(6):1968-1977.
doi - https://www.acr.org/-/media/ACR/Files/Clinical-Resources/LIRADS/LI-RADS-2018-Manual-5Dec18.pdf.
- ECOG Performance Status Scale. Available at: https://ecog-acrin.org/resources/ecog-performance-status/. Accessed June 28, 2022.
- Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334(11):693-699.
doi - Mehta N, Frenette C, Tabrizian P, Hoteit M, Guy J, Parikh N, Ghaziani TT, et al. Downstaging outcomes for hepatocellular carcinoma: results from the multicenter evaluation of reduction in tumor size before liver transplantation (MERITS-LT) consortium. Gastroenterology. 2021;161(5):1502-1512.
doi - Mazzaferro V, Citterio D, Bhoori S, Bongini M, Miceli R, De Carlis L, Colledan M, et al. Liver transplantation in hepatocellular carcinoma after tumour downstaging (XXL): a randomised, controlled, phase 2b/3 trial. Lancet Oncol. 2020;21(7):947-956.
doi - Hameed B, Mehta N, Sapisochin G, Roberts JP, Yao FY. Alpha-fetoprotein level > 1000 ng/mL as an exclusion criterion for liver transplantation in patients with hepatocellular carcinoma meeting the Milan criteria. Liver Transpl. 2014;20(8):945-951.
doi - Mazzaferro V, Sposito C, Zhou J, Pinna AD, De Carlis L, Fan J, Cescon M, et al. Metroticket 2.0 Model for Analysis of Competing Risks of Death After Liver Transplantation for Hepatocellular Carcinoma. Gastroenterology. 2018;154(1):128-139.
doi - Duvoux C, Roudot-Thoraval F, Decaens T, Pessione F, Badran H, Piardi T, Francoz C, et al. Liver transplantation for hepatocellular carcinoma: a model including alpha-fetoprotein improves the performance of Milan criteria. Gastroenterology. 2012;143(4):986-994.e983.
doi - https://www.chronicliverdisease.org/disease_focus/hcc.cfm?dstate=hcc&sec=Algorithm.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


