| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Original Article
Volume 15, Number 2, April 2022, pages 82-90
Burden and Predictors of Non-Alcoholic Fatty Liver Disease in a Retrospective Cohort of Patients With Crohn’s Disease
Ahmed Abomhyaa, c , Mohammed Mahmoodurrahmana, Salman Ayaza, Hossam Hamadb, Farrah Khana
aDepartment of Internal Medicine, The Brooklyn Hospital Center, Brooklyn, NY, USA
bFaculty of Medicine, Al-Azhar University, Cairo, Egypt
cCorresponding Author: Ahmed Abomhya, Department of Internal Medicine, The Brooklyn Hospital Center, Brooklyn, NY, USA
Manuscript submitted February 13, 2022, accepted March 16, 2022, published online April 12, 2022
Short title: Predictors of NAFLD in Crohn’s Disease
doi: https://doi.org/10.14740/gr1509
| Abstract | ▴Top |
Background: Non-alcoholic fatty liver disease (NAFLD) is an emerging extraintestinal manifestation (EIM) of Crohn’s disease (CD). We aimed to investigate the prevalence and comorbid predictors of NAFLD in patients with CD.
Methods: We conducted a nationwide retrospective cohort study to determine the prevalence, characteristics, comorbidities, and hospitalization outcomes associated with NAFLD in patients with CD. Comparison between groups was performed by Mann-Whitney test for continuous variables and Chi-square test for categorical variables. We performed a binary logistic regression analysis for predictors of NAFLD among patients with CD.
Results: We extracted 215,049 index hospital discharges with CD; 2.4% had NAFLD. CD patients, with NAFLD, had increased length of stay (4 days; interquartile range (IQR): 2 - 6 vs. 3; IQR: 2 - 6, P < 0.01), and increased median total charges ($32,305.5; IQR: $18,600 - $61,599 vs. $30,782; IQR: $16,847 - $58,667, P < 0.01), compared to CD patients without NAFLD. Non-alcoholic steatohepatitis (NASH) was found to be independently associated with increased mortality (odds ratio (OR): 1.7; 95% confidence interval (CI): 1.1 - 2.6, P = 0.03) and a higher odd for all-cause 30-day non-elective readmission (OR: 1.6: 95% CI: 1.3 - 1.9, P < 0.001). Factors independently associated with NAFLD in patients with CD included portal hypertension (OR: 5.347; 95% CI: 4.604 - 6.211, P < 0.001), vitamin A deficiency (OR: 9.89; 95% CI: 4.49 - 21.76, P < 0.001) and vitamin B12 deficiency (OR: 1.56; 95% CI: 1.098 - 2.209, P = 0.013).
Conclusions: NAFLD is associated with worse hospitalization outcomes in patients with CD. Study findings suggest the need for early identification and effective management of NAFLD predictors to reduce complications.
Keywords: Non-alcoholic fatty liver disease; Crohn’s disease; Steatosis; Non-alcoholic steatohepatitis; Vitamin B12 deficiency
| Introduction | ▴Top |
Background
Non-alcoholic fatty liver disease (NAFLD) is characterized by fatty accumulations in hepatocytes in the absence of other secondary causes of steatosis [1]. Non-alcoholic steatohepatitis (NASH) results from the progression of benign steatosis and can potentially progress and lead to cirrhosis, hepatocellular carcinoma, and death [2]. NAFLD has now grown to be the most prominent cause of chronic liver disease worldwide [3], and the overall global prevalence of NAFLD is estimated to be around 25% [4]. The prevalence of NASH is estimated to be between 1.5% and 6.5% [4].
Crohn’s disease (CD) is an inflammatory bowel disease (IBD) that affects the gastrointestinal tract in a non-contiguous manner with transmural inflammation [5]. The annual incidence of CD in North America is reported to be around 3.1 - 20.2 per 100,000, with a prevalence of 201 per 100,000 population [6]. In most cases, CD has a chronic and intermittent course. About 20% of patients achieve remission after the initial diagnosis [7]. Chronic bowel inflammation can lead to the development of intestinal complications and multiple extraintestinal manifestations.
The pathophysiology of NAFLD has been extensively studied, and multiple factors have been associated with NAFLD [8], including inflammatory cytokines [9], inflammasomes [10], mitochondrial dysfunction [11, 12], and genetic and epigenetic factors [8, 13-15]. NAFLD is the most common cause of abnormal liver function test (LFT) in IBD patients [16]. Murine models of IBD have shown an association between IBD and NAFLD. Kwon et al showed that colitis causes a disturbance in hepatic fatty acid oxidation and reverse cholesterol transport [17]. Current data suggest that the prevalence of NAFLD in patients with IBD is between 8% and 71% [18].
There is a significant healthcare cost associated with CD. The cost of care incurred by patients with IBD is three times higher when compared with non-IBD patients [19]. Moreover, there are data suggesting that the average healthcare cost for CD patients has exceeded most of the previously reported estimates [20]. NAFLD is associated with significant humanistic and economic burdens. There are more than 64 million patients with NAFLD in the USA, with an associated cost of over a hundred billion annually [21].
Objectives
In this retrospective cohort study, we studied the prevalence, outcomes, and predictors of NAFLD in patients with CD. The primary study outcomes were the prevalence of NAFLD in a nationwide cohort of CD patients, and hospitalization outcomes associated with NAFLD including length of stay (LOS) in days, all-cause 30-day non-elective readmission, and inpatient mortality. The secondary outcome was identifying the predictors independently associated with a diagnosis of NAFLD in patients with CD. Subgroup analysis was performed for hospitalization outcomes of CD patients, with and without NASH.
| Materials and Methods | ▴Top |
Study design and setting
This is a retrospective cohort study of CD patients discharged from US hospitals between 2016 and 2018. The Nationwide Readmissions Database (NRD) is a publicly available all-payer inpatient database in the USA sponsored by the Agency for Healthcare Research and Quality (AHRQ) through a Federal-State-Industry partnership [22]. The NRD contains patient linkage numbers to track patients across hospitals within a state. NRD includes discharge data that represent 60.4% of all US hospitalizations. The use of the NRD is exempt from an Institutional Review Board, as there is no identifying patient information [23, 24]. The study was performed in accordance with the 1963 Helsinki Declaration, its later amendments, and comparable ethical standards.
Participants
All hospitalizations for CD in NRD (2016 - 2018) were used for the cohort study. Patients with a comorbid diagnosis of NAFLD (including NASH) were identified. We excluded patients younger than18 years old, those with missing principal diagnoses, or missing information (Fig. 1). The diagnoses were reported using the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes (Supplementary Material 1, www.gastrores.org).
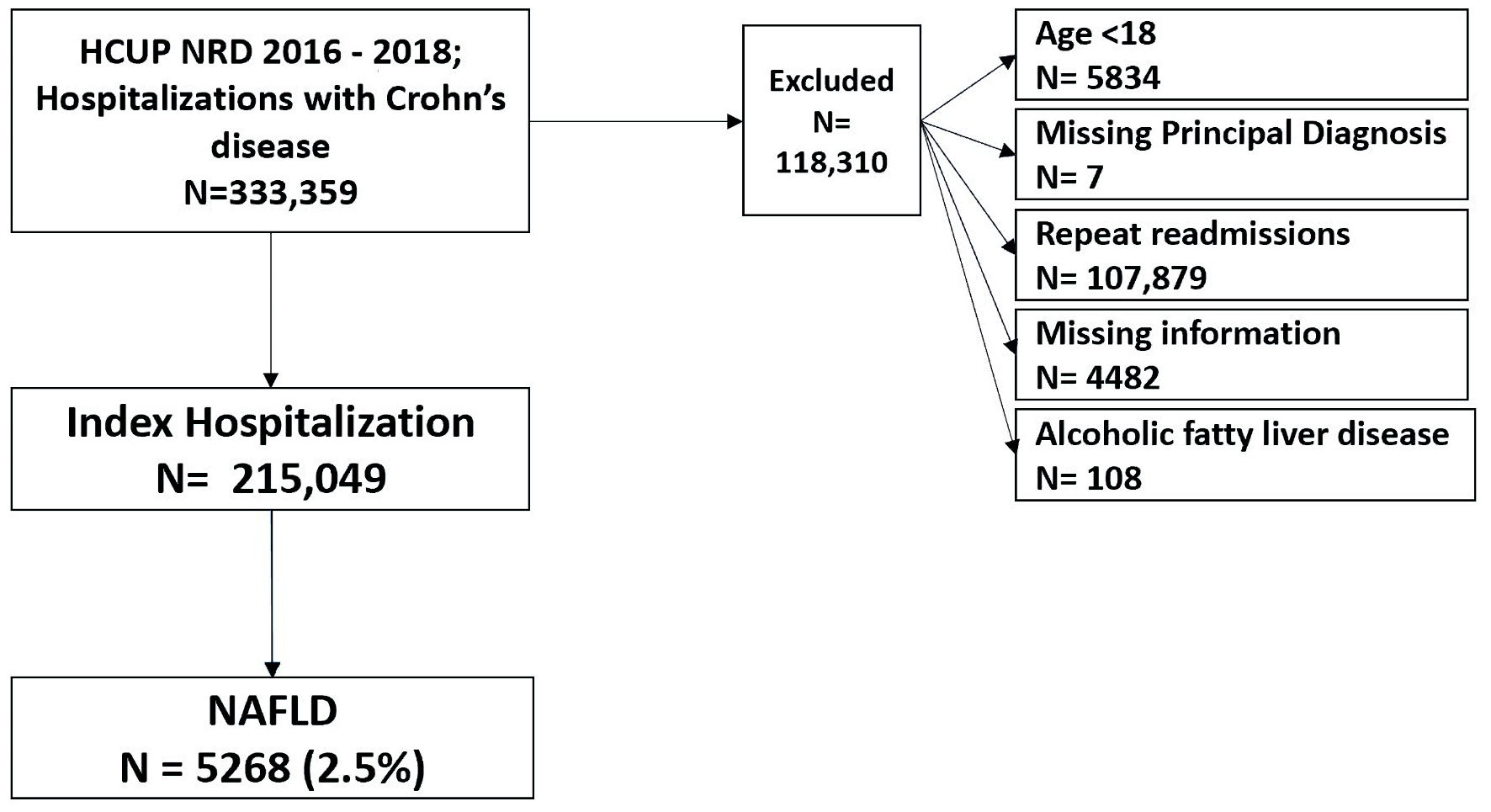 Click for large image | Figure 1. Case selection flowchart. NAFLD: non-alcoholic fatty liver disease; HCUP: Healthcare Cost and Utilization Project; NRD: Nationwide Readmissions Database. |
Variables
We identified patients’ characteristics, including demographic factors, hospitalization factors, and comorbidities. Demographic factors included were age, sex, income status, and patient location. Hospitalization factors included were elective admission, bed size of the hospital, and insurance type.
Comorbidities investigated included metabolic syndrome, diabetes mellitus, obesity, hypertension, dyslipidemia, vitamin deficiencies (A, D, E, K, B12 and pyridoxine), abnormal LFT, chronic pancreatitis, thrombocytopenia, portal hypertension, gallstone disease and Clostridium difficile enterocolitis.
Index hospitalization discharges occurring in December were excluded from the 30-day non-elective readmission analysis as readmissions were captured purely on a calendar year. Elective readmissions and patients who died in the index hospitalization were excluded from the readmission analysis too.
After the individuals with subsequent readmission were identified, readmission records were excluded and only first index hospitalization records were included in the analysis to ensure independence between observations before running the regression model.
Statistical analysis
The NRD data were extracted and analyzed using SPSS Version 25 (IBM Corporation, Armonk, NY, USA). Numeric variables were tested for normality using Kolmogorov-Smirnov test. Nonparametric numeric variables were described with median and interquartile range (IQR) and compared with the Mann-Whitney test. Categorical variables were described with percentages and compared using Chi-square tests. A P value < 0.05 was considered statistically significant. The factors significant in univariate analyses at P < 0.05 were included in the final binary logistic regression analysis (forward method) to identify predictors of NAFLD among patients with CD. The causes of unplanned readmissions were examined with a table of rates.
| Results | ▴Top |
Baseline characteristics of CD patients with and without NAFLD
We extracted 333,359 hospital discharge records with a diagnosis of CD or one of its complications. We excluded records with missing a principal diagnosis, missing information, age < 18, a diagnosis of alcoholic fatty liver disease, or readmission records. We extracted 215,049 index hospital discharges with a diagnosis of CD. NAFLD was present in 2.4% of CD patients, while NASH was present in 0.42% of CD patients. There was no significant difference in median age between CD patients with and without NAFLD (53; IQR: 36 - 68 vs. 53; IQR: 42 - 63, respectively). The baseline characteristics of CD patients with NAFLD are summarized in Table 1.
 Click to view | Table 1. Baseline Characteristics of CD Patients With and Without NAFLD |
Comorbid diseases among CD patients with and without NAFLD
There was a significant increase in the prevalence of diabetes mellitus (28.5% vs. 15.2%, P < 0.001), obesity (29.3% vs. 11.3%, P < 0.001), hypertension (41.5% vs. 29.8%, P < 0.001), metabolic syndrome (0.7% vs. 0.1%, P < 0.001), and dyslipidemia (28.2% vs. 21.1%, P < 0.001) in CD patients with NAFLD, when compared with CD patients without NAFLD, respectively (Table 2).
 Click to view | Table 2. Comorbid Diseases Among CD Patients With and Without NAFLD |
While 64% of CD patients with NAFLD had at least one of the five metabolic risk factors for NAFLD (hypertension, diabetes mellitus, dyslipidemia, obesity, and metabolic syndrome), 35.9% of CD patients with NAFLD had no records of any of those conditions.
There was a significant increase in the prevalence of vitamin A deficiency (0.2% vs. 0.01%, P < 0.001), vitamin D deficiency (4.9% vs. 3.1%, P < 0.001), vitamin E deficiency (0.1% vs. 0.01%, P = 0.009), and vitamin B12 deficiency (0.6% vs. 0.4%, P = 0.026) in CD patients with NAFLD, when compared to CD patients without NAFLD, respectively.
CD patients with NAFLD had a higher prevalence of abnormal LFT (0.6% vs. 0.2%, P < 0.001), thrombocytopenia (9.4% vs. 3.6%, P < 0.001), gallstone disease (4.2% vs. 1.1%, P < 0.001), chronic pancreatitis (2.7% vs. 0.8%, P < 0.001), Clostridium difficile enterocolitis (3.6% vs. 2.6%, P < 0.001), and portal hypertension (5% vs. 0.6%, P < 0.001) compared to CD patients without NAFLD. In our cohort, we found that 10.2% of CD patients with NAFLD have developed cirrhosis.
Gastrointestinal complications among CD patients with and without NAFLD
NAFLD was more prevalent among CD patients without gastrointestinal complications (2.5% vs. 2.3%, P< 0.001) compared to CD patients with gastrointestinal complications.
We analyzed the prevalence of gastrointestinal complications including CD with fistula, CD with intestinal obstruction, CD with abscess, and CD with rectal bleeding in CD patients with NAFLD (Table 3). CD patients with NAFLD had a higher prevalence of rectal bleeding (5.1% vs. 3.9%, P < 0.001) compared to CD patients without NAFLD.
 Click to view | Table 3. Gastrointestinal Complications Among CD Patients With and Without NAFLD |
Hospitalization outcomes for CD patients with and without NAFLD
CD patients with NAFLD had a significantly increased LOS (4; IQR: 2 - 6 vs. 3; IQR: 2 - 6, P < 0.001), and increased total charges ($32,305.5; IQR: $18,600 - $61,599 vs. $30,782; IQR: $16,847 - $58,667, P < 0.001) compared to CD patients without NAFLD, respectively (Table 4).
 Click to view | Table 4. Hospitalization Outcomes for CD Patients With and Without NAFLD |
Subgroup analysis of hospitalization outcomes for CD patients, with and without NASH, showed a significantly increased mortality (2.2% vs. 1.2%, P = 0.004), increased LOS (4; IQR: 2 - 6 vs. 3; IQR: 2 - 6, P < 0.001), and a higher rate of 30-day non-elective readmission (15.6% vs. 11.1%, P < 0.001) in CD patients with NASH, compared to CD patients without NASH, respectively (Table 5).
 Click to view | Table 5. Hospitalization Outcomes for CD Patients With and Without NASH |
In multivariate analysis, after adjusting for age, gender, CD complications, and comorbid conditions (hypertension, diabetes mellitus, dyslipidemia, obesity, and metabolic syndrome), NASH was found to be independently associated with increased mortality (OR: 1.7; 95% CI: 1.1 - 2.6, P = 0.03) and a higher odd for all-cause 30-day non-elective readmission (OR: 1.6; 95% CI: 1.3 - 1.9, P < 0.001).
Most common causes of 30-day readmission among CD patients with NASH
We analyzed the most encountered causes for 30-day non-elective readmissions in CD patients with NASH. Sepsis, hepatic failure with coma, acute kidney injury (AKI), and CD were the most encountered primary readmission diagnoses. A list of the most common readmission primary diagnosis is illustrated in Table 6.
 Click to view | Table 6. Most Common Causes of 30-Day Readmission Among CD Patients With NASH |
Logistic regression model for factors associated with NAFLD in patients with CD
Vitamin A deficiency (OR: 9.89; 95% CI: 4.49 - 21.76, P < 0.001), abnormal LFT (OR: 3.29; 95% CI: 2.29 - 4.72, P < 0.001), metabolic syndrome (OR: 2.69; 95% CI: 1.84 - 3.94, P < 0.001), obesity (OR: 2.84; 95% CI: 2.65 - 3.04, P < 0.001), diabetes mellitus (OR: 1.81; 95% CI: 1.68 - 1.96, P < 0.001), vitamin D deficiency (OR: 1.43; 95% CI: 1.25 - 1.63, P < 0.001), hypertension (OR: 1.45; 95% CI: 1.41 - 1.6, P < 0.001), vitamin B12 deficiency (OR: 1.56; 95% CI: 1.1 - 2.21, P = 0.013) CD with rectal bleeding (OR: 1.41; 95% CI: 1.16 - 1.49, P < 0.001), gallstone disease (OR: 3.44; 95% CI: 2.97 - 3.99, P < 0.001), chronic pancreatitis (OR: 2.73; 95% CI: 2.28 - 3.27, P < 0.001), thrombocytopenia (OR: 2.14; 95% CI: 1.93 - 2.38, P < 0.001), Clostridium difficile infection (OR: 1.41; 95% CI: 1.22 - 1.64, P < 0.001), portal hypertension (OR: 5.35; 95% CI: 4.6 - 6.21, P < 0.001), age group 45 - 64 (OR: 1.23; 95% CI: 1.14 - 1.32, P < 0.001), and dyslipidemia (OR: 1.29; 95% CI: 1.2 - 1.39, P < 0.01) were positive and significant predictors for NAFLD in CD patients.
CD with fistula (OR: 0.76; 95% CI: 0.63 - 0.91, P = 0.004) was a negative predictor of NAFLD in patients with CD. Predictors of NAFLD in patients with CD are illustrated in Table 7.
 Click to view | Table 7. Logistic Regression Model for Factors Associated With NAFLD in Patients With CD |
| Discussion | ▴Top |
NAFLD is the most common chronic liver disease in Western countries [25]. NAFLD was present in 2.5% of patients with CD in our cohort. McHenry et al had reported a prevalence of 38% in his prospective study, in which he used magnetic resonance proton density fat fraction (MR-PDFF) maps to screen for NAFLD in patients with IBD [26]. The large variation in the reported prevalence rates of NAFLD could be attributed to the differences in the sample size, NAFLD diagnostic criteria, and the respective study designs.
Given the higher number of CD patients with NAFLD included in our study, we had the opportunity to study the comorbid predictors of NAFLD in patients with CD. Around one-third of CD patients with NAFLD analyzed in our study had no records of metabolic syndrome components (diabetes mellitus, hypertension, obesity, dyslipidemia), suggesting that CD is an independent risk factor for NAFLD.
Prior studies have proposed the role of micronutrient deficiencies in the development of NAFLD in patients with CD [27-29]. We showed a clear association between the deficiency of vitamins A, E, and D and NAFLD in patients with CD. In addition, we also report a novel finding of a significant association between vitamin B12 deficiency and NAFLD in patients with CD. Multiple studies are currently evaluating the therapeutic role of vitamin supplements in patients with NAFLD. Our study demonstrates the association between NAFLD and multiple micronutrient deficiencies and provides novel insights into potential therapeutic candidates.
There was a significantly higher risk of rectal bleeding in CD patients with NAFLD compared to CD patients without NAFLD, which might be related to the higher prevalence of thrombocytopenia and portal hypertension in CD patients with NAFLD in our cohort. More than one in 10 NAFLD patients in our cohort had cirrhosis, which can explain the higher prevalence of portal hypertension, thrombocytopenia, and rectal bleeding in this group. In addition, we highlight the higher prevalence of Clostridium difficile enterocolitis among CD patients with NAFLD, which is another remarkable finding.
Our study also showed that patients with NASH had a higher all-cause 30-day non-elective readmission rate compared to CD patients without NASH. Sepsis was the most common primary cause of 30-day readmissions among CD patients with NASH. These findings support prior studies that reported that NAFLD is an independent risk factor for sepsis [30]. NAFLD is associated with an increased long-term risk of gastrointestinal cancers [31]. Future studies are needed to evaluate the incidence and risk of cancer in CD patients with a comorbid diagnosis of NAFLD.
Our study does have some limitations. Firstly, an NRD file does not track patients across years or across different states, which decreases the condition-specific readmission rates by a margin of 5% or less for most of the Clinical Classifications Software (CCS) categories [32]. Secondly, the use of administrative codes is subject to misclassification. Lastly, NRD does not provide data on alcohol use, prescribed drugs, and we were unable to adjust for the effect of medications that can alleviate or aggravate NAFLD including but not limited to steroids, statins, various anti-diabetic agents, and various anti-hypertensive agents. However, our study has several strengths. This is the first nationwide study to evaluate the association between CD and NAFLD. We used a national database, which accounts for 60.4% of all hospitalizations in the USA. The NRD has been validated for use in clinical and epidemiological research studies.
In conclusion, our study showed that NAFLD is a common comorbidity in patients with CD and a significant determinant of morbidity and mortality. We reported multiple factors independently associated with NAFLD in patients with CD including vitamin B12 deficiency, thrombocytopenia, vitamin A deficiency, vitamin D deficiency, chronic pancreatitis, Clostridium difficile enterocolitis, and portal hypertension. Further studies are needed to investigate the pathogenesis and potential therapeutic of NAFLD among patients with CD.
| Supplementary Material | ▴Top |
Suppl 1. ICD-10-CM codes used in this study.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
No consent was required as the NRD database lacks patient-specific identifiers.
Author Contributions
Conception and design of the study: Ahmed Abomhya. Acquisition, analysis, interpretation, and statistical analysis of data: all authors. Literature search and review: all authors. Writing and revising the manuscript critically for important intellectual content: all authors. Approval of the final version of the manuscript: all authors. Agreement to be accountable for all aspects of the study: all authors.
Data Availability
The NRD is available at: https://www.hcup-us.ahrq.gov/nrdoverview.jsp. The data supporting our findings are available within the article.
Abbreviations
CD: Crohn’s disease; NAFLD: non-alcoholic fatty liver disease; HCUP: Healthcare Cost and Utilization Project; NRD: Nationwide Readmissions Database; ICD-10-CM: International Classification of Diseases, Tenth Revision, Clinical Modification; LOS: length of stay; IQR: interquartile range; OR: odds ratio; CI: confidence interval; CCS: Clinical Classifications Software; NASH: non-alcoholic steatohepatitis; LFT: liver function test; MR-PDFF: magnetic resonance proton density fat fraction
| References | ▴Top |
- Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, Natale S, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37(4):917-923.
doi pubmed - Sanyal AJ, Brunt EM, Kleiner DE, Kowdley KV, Chalasani N, Lavine JE, Ratziu V, et al. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology. 2011;54(1):344-353.
doi pubmed - Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15(1):11-20.
doi pubmed - Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73-84.
doi pubmed - Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328-357.
doi pubmed - Kappelman MD, Rifas-Shiman SL, Kleinman K, Ollendorf D, Bousvaros A, Grand RJ, Finkelstein JA. The prevalence and geographic distribution of Crohn's disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5(12):1424-1429.
doi pubmed - Solberg IC, Vatn MH, Hoie O, Stray N, Sauar J, Jahnsen J, Moum B, et al. Clinical course in Crohn's disease: results of a Norwegian population-based ten-year follow-up study. Clin Gastroenterol Hepatol. 2007;5(12):1430-1438.
doi pubmed - Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. 2016;65(8):1038-1048.
doi pubmed - Tomita K, Tamiya G, Ando S, Ohsumi K, Chiyo T, Mizutani A, Kitamura N, et al. Tumour necrosis factor alpha signalling through activation of Kupffer cells plays an essential role in liver fibrosis of non-alcoholic steatohepatitis in mice. Gut. 2006;55(3):415-424.
doi pubmed - Wree A, McGeough MD, Pena CA, Schlattjan M, Li H, Inzaugarat ME, Messer K, et al. NLRP3 inflammasome activation is required for fibrosis development in NAFLD. J Mol Med (Berl). 2014;92(10):1069-1082.
doi pubmed - Paradies G, Paradies V, Ruggiero FM, Petrosillo G. Oxidative stress, cardiolipin and mitochondrial dysfunction in nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20(39):14205-14218.
doi pubmed - Begriche K, Igoudjil A, Pessayre D, Fromenty B. Mitochondrial dysfunction in NASH: causes, consequences and possible means to prevent it. Mitochondrion. 2006;6(1):1-28.
doi pubmed - Wilfred de Alwis NM, Day CP. Genetics of alcoholic liver disease and nonalcoholic fatty liver disease. Semin Liver Dis. 2007;27(1):44-54.
doi pubmed - Podrini C, Borghesan M, Greco A, Pazienza V, Mazzoccoli G, Vinciguerra M. Redox homeostasis and epigenetics in non-alcoholic fatty liver disease (NAFLD). Curr Pharm Des. 2013;19(15):2737-2746.
doi pubmed - Pogribny IP, Tryndyak VP, Bagnyukova TV, Melnyk S, Montgomery B, Ross SA, Latendresse JR, et al. Hepatic epigenetic phenotype predetermines individual susceptibility to hepatic steatosis in mice fed a lipogenic methyl-deficient diet. J Hepatol. 2009;51(1):176-186.
doi pubmed - Cappello M, Randazzo C, Bravata I, Licata A, Peralta S, Craxi A, Almasio PL. Liver function test abnormalities in patients with inflammatory bowel diseases: a hospital-based survey. Clin Med Insights Gastroenterol. 2014;7:25-31.
doi pubmed - Kwon J, Lee C, Heo S, Kim B, Hyun CK. DSS-induced colitis is associated with adipose tissue dysfunction and disrupted hepatic lipid metabolism leading to hepatosteatosis and dyslipidemia in mice. Sci Rep. 2021;11(1):5283.
doi pubmed - Karaivazoglou K, Konstantakis C, Tourkochristou E, Assimakopoulos SF, Triantos C. Non-alcoholic fatty liver disease in inflammatory bowel disease patients. Eur J Gastroenterol Hepatol. 2020;32(8):903-906.
doi pubmed - Park KT, Ehrlich OG, Allen JI, Meadows P, Szigethy EM, Henrichsen K, Kim SC, et al. The cost of inflammatory bowel disease: an initiative from the Crohn's & colitis foundation. Inflamm Bowel Dis. 2020;26(1):1-10.
doi pubmed - Park KT, Colletti RB, Rubin DT, Sharma BK, Thompson A, Krueger A. Health insurance paid costs and drivers of costs for patients with Crohn's disease in the United States. Am J Gastroenterol. 2016;111(1):15-23.
doi pubmed - Younossi ZM, Henry L. Economic and quality-of-life implications of non-alcoholic fatty liver disease. Pharmacoeconomics. 2015;33(12):1245-1253.
doi pubmed - HCUP-US NRD Overview. Accessed September 21, 2021. Available from: https://www.hcup-us.ahrq.gov/nrdoverview.jsp.
- Rothstein MA. Is deidentification sufficient to protect health privacy in research? Am J Bioeth. 2010;10(9):3-11.
doi pubmed - The HHS regulations for the protection of human subjects in research at 45CFR 46. Accessed November 21, 2020. Available from: https://www.hhs.gov/ohrp/regulations-and-policy/regulations/45-cfr-46/index.html.
- Carr RM, Oranu A, Khungar V. Nonalcoholic Fatty Liver Disease: Pathophysiology and Management. Gastroenterol Clin North Am. 2016;45(4):639-652.
doi pubmed - McHenry S, Sharma Y, Tirath A, Tsai R, Mintz A, Fraum TJ, Salter A, et al. Crohn's disease is associated with an increased prevalence of nonalcoholic fatty liver disease: a cross-sectional study using magnetic resonance proton density fat fraction mapping. Clin Gastroenterol Hepatol. 2019;17(13):2816-2818.
doi pubmed - Targher G, Bertolini L, Scala L, Cigolini M, Zenari L, Falezza G, Arcaro G. Associations between serum 25-hydroxyvitamin D3 concentrations and liver histology in patients with non-alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2007;17(7):517-524.
doi pubmed - Jablonski KL, Jovanovich A, Holmen J, Targher G, McFann K, Kendrick J, Chonchol M. Low 25-hydroxyvitamin D level is independently associated with non-alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2013;23(8):792-798.
doi pubmed - Liu Y, Chen H, Wang J, Zhou W, Sun R, Xia M. Association of serum retinoic acid with hepatic steatosis and liver injury in nonalcoholic fatty liver disease. Am J Clin Nutr. 2015;102(1):130-137.
doi pubmed - Hou J, Zhang J, Cui P, Zhou Y, Liu C, Wu X, Ji Y, et al. TREM2 sustains macrophage-hepatocyte metabolic coordination in nonalcoholic fatty liver disease and sepsis. J Clin Invest. 2021;131(4):e135197.
doi pubmed - Mantovani A, Petracca G, Beatrice G, Csermely A, Tilg H, Byrne CD, Targher G. Non-alcoholic fatty liver disease and increased risk of incident extrahepatic cancers: a meta-analysis of observational cohort studies. Gut. 2022;71(4):778-788.
doi pubmed - HCUP-US NRD Introduction. Accessed September 21, 2021. Available from: https://www.hcup-us.ahrq.gov/db/nation/nrd/Introduction_NRD_2010-2018.pdf.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


