| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Original Article
Volume 14, Number 5, October 2021, pages 259-267
The Incidence of Endoscopic Retrograde Cholangiopancreatography-Related Complications in Patients With Liver Transplant: A Meta-Analysis and Systematic Review
Mohammad Alomaria, Laith Al Momanib, Ahmed Alomaric, Shrouq Khazaalehd, Asif Ali Hitawalad, Amani Khasawnehe, Prashanthi N. Thotaf, Sreeni S. Jonnalagaddab, Madhusudhan R. Sanakaf, g
aDepartment of Gastroenterology and Hepatology, Cleveland Clinic Florida, Weston, FL, USA
bDepartment of Gastroenterology and Hepatology, University of Missouri - Kansas City, Kansas City, MO, USA
cDepartment of Gastroenterology and Hepatology, University Hospitals Southampton NHS Trust, Southampton, England, UK
dDepartment of Internal Medicine, Cleveland Clinic Fairview Hospital, Cleveland, OH, USA
eDepartment of Internal Medicine, King Abdullah University Hospital, Irbid, Jordan
fDepartment of Gastroenterology, Hepatology and Nutrition, Cleveland Clinic Foundation, Cleveland, OH, USA
gCorresponding Author: Madhusudhan R. Sanaka, Department of Gastroenterology, Hepatology and Nutrition, Cleveland Clinic Foundation, 9500 Euclid Avenue, Cleveland, OH 44195, USA
Manuscript submitted April 11, 2021, accepted September 1, 2021, published online September 15, 2021
Short title: Post-ERCP Complications in LT Patients
doi: https://doi.org/10.14740/gr1391
| Abstract | ▴Top |
Background: Existing literature on post-endoscopic retrograde cholangiopancreatography (ERCP) complications in patients with liver transplant remains scarce and largely inconsistent. We therefore aimed to systematically review and analyze the literature on complication rates associated with ERCP in patients with liver transplant.
Methods: We performed a comprehensive literature search in PubMed, PubMed Central, Embase, and ScienceDirect databases from inception through March 2020 to identify all the studies that evaluated post-ERCP complications in patients with liver transplant. Effect estimates from the individual studies were extracted and combined using the random effect, generic inverse variance method of DerSimonian and Laird, and a pooled odds ratio (OR) and event rates were calculated. Forest plots were generated, and publication bias was assessed for using conventional techniques.
Results: Fourteen studies with a total of 1,787 patients were analyzed. In total, 3,192 ERCPs were performed on these patients. The pooled all-complication rate was 5.2% (95% confidence interval (CI): 0.035 - 0.075). Procedural complications analyzed included post-ERCP pancreatitis 3.4% (95% CI: 0.025 - 0.047), bleeding 1.1% (95% CI: 0.006 - 0.020), infections 0.2% (95% CI: 0.025 - 0.047), and cholangitis 0.8% (95% CI: 0.004 - 0.020). No cases of periprocedural death were reported. The pooled OR for post-ERCP pancreatitis in patients with liver transplant compared to patients without liver transplant was 1.289 (95% CI: 0.455 - 3.653, P = 0.633, I2 = 72.88%).
Conclusion: Post-ERCP complication rates in liver transplant patients are comparable to the general population and hence, peri-procedural evaluation and management may follow the current standards of care in this patient population.
Keywords: Liver transplant; Endoscopic retrograde cholangiopancreatography; Complications; Pancreatitis and cholangitis
| Introduction | ▴Top |
Ever since the first liver transplantation was performed by Thomas Starzl in 1963, the world has witnessed progressive advances in transplant techniques, immunosuppression, and patient selection. Undoubtedly, this has revolutionized the management of end-stage liver disease (ESLD). In the last two decades, the total number of liver transplants performed, nationally and internationally, has continued to climb [1, 2], approaching 30,000 transplants worldwide in 2018 alone [1, 2].
The advent of the Model for End-Stage Liver Disease (MELD) with subsequent increased liver transplant eligibility of higher risk patients, and the increased post-transplant survival necessitated a particular attention to liver transplant-related complications. Historically, causes of post-transplantation deaths were divided into hepatic and non-hepatic causes, with hepatic causes accounting for 23.9% of deaths [3]. The most common post liver transplant biliary complications include leaks, anastomotic and non-anastomotic strictures, as well as biliary obstruction with casts, stones, and sludge [4, 5].
Although recent evolutions in liver transplant surgical techniques have reduced the complication rates, post-operative biliary complications remain an important cause of morbidity and often require surgical revision. Endoscopic retrograde cholangiopancreatography (ERCP) has proven to be an indispensable tool for the management of some of these complications, including biliary strictures and bile leaks [6-8]. ERCP, however, is not without its own set of risks and complications, including pancreatitis, bleeding, infection, and perforation [5].
The reported incidence of post-ERCP complications varies widely amongst transplant patients. Some studies have shown overall ERCP complication rates to be fairly similar between liver transplant patients and the general population [5, 9]. However, there have been conflicting data regarding the prevalence of these specific complications [5, 9].
Despite the emerging body of literature examining the epidemiology of post-ERCP complications in liver transplant patients, the results of these studies remain inconsistent. Hence, we conducted this meta-analysis to systematically review and analyze the literature on complication rates associated with ERCP in patients with liver transplant.
We present the following article in accordance with the PRISMA reporting checklist.
| Materials and Methods | ▴Top |
Search strategy and selection criteria
A comprehensive literature search was performed in PubMed, PubMed Central, Embase, and ScienceDirect databases from inception through March 2020 to identify all of the studies that evaluated post-ERCP complications in patients with liver transplant. Keywords used included liver transplant, ERCP, complications, adverse events, infections, pancreatitis, cholangitis, bleeding, and post-transplant. The search was limited to human studies with no restrictions placed on region, publication type, or language.
Data extraction and quality assessment
To be included studies were required to meet the following criteria: 1) Implemented a well-defined case-control or cohort design; and 2) Either presented an odds ratio (OR) or an event rate for our main outcome with a 95% confidence interval (CI), or presented the data sufficient to calculate the event rate or OR. Studies were excluded for the following reasons: 1) They were letters to the editor/authors, case reports, case series, or review articles; or 2) Provided insufficient information to calculate the OR or event rate for our main outcome. The data from the included studies were entered into a standardized table for analysis. The quality of included studies was assessed independently by two authors using the Newcastle-Ottawa scale [10] for observational studies. A third author addressed any discrepancy by joint evaluation of the original article.
Statistical analysis
Statistical analysis was performed using the Comprehensive Meta-Analysis (CMA), Version 3 software (BioStat, Inc., Eaglewood, NJ). Effect estimates from the individual studies were extracted and combined using the random-effect, generic inverse variance method of DerSimonian and Laird [11]. A random effect model was used as a high probability of between-study variance was suspected due to variation in study population and methodology. A pooled OR was calculated. A Cochran’s Q-test and an I2 statistic were used to evaluate heterogeneity and quantify variation across the selected studies. A funnel plot was then created to evaluate for publication and other reporting biases and then the plot was examined visually for asymmetry. Finally, an Egger test for asymmetry of a funnel plot was conducted.
Ethics approval is not required by our institution to conduct or publish systematic reviews and/or meta-analyses.
This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
| Results | ▴Top |
Search results
The initial comprehensive search yielded 746 citations. All citations underwent a title and abstract review, with the majority being excluded for either being duplicates, letters to the editor, case reports, or case series. Of the initial yield, 57 citations underwent a full-length article review, and 43 were excluded as they did not include controls, were review articles, or did not provide sufficient information to calculate the event rate and/or the OR for our main outcome. A flow diagram illustrates the selection process (Fig. 1). Subsequently, a total of 14 studies met the inclusion criteria and were included in this analysis.
 Click for large image | Figure 1. Flow diagram illustrating the selection process. |
Characteristics of included studies
The characteristics of the studies used in the meta-analysis are shown in Table 1 [5, 12-24].
 Click to view | Table 1. Summary of the Studies Used in Meta-Analysis |
Husing et al performed a retrospective single-center study conducted in Munster, Germany. A total of 454 ERCPs with endoscopic sphincterotomy in 157 liver transplant recipients were reviewed for complications [5]. Ambrus et al performed a retrospective study in Copenhangen, Denmark in which complications from 292 ERCPs were reviewed in 127 patients with liver transplant [12]. Law et al performed a multicenter retrospective analysis of complications from 730 ERCPs in 301 patients with liver transplant at both Mayo Clinic (Rochester, MN) and Hospital Clinic (Barcelona, Spain) [13]. Sanna et al retrospectively reviewed complications after 150 ERCP procedures in a total of 94 liver transplant patients in Torino, Italy [14]. Balderramo et al performed a retrospective evaluation of complications from 243 ERCPs in 121 patients with liver transplant in Barcelona, Spain [15]. Pievsky et al retrospectively reviewed complications after 219 ERCP procedures in 120 liver transplant recipients in New Jersey [16]. Singh et al performed a retrospective study in which complications from 159 ERCPs in 71 liver transplant patients were reviewed [17]. Brown et al conducted a retrospective study at the University of Cincinnati Medical Center in which a total of 98 ERCPs in 98 liver transplant patients treated with tacrolimus were evaluated for post-ERCP pancreatitis (PEP) [18]. Abu Rajab and Gan retrospectively reviewed 146 ERCPs in 146 liver transplant recipients at Tufts-New England Medical Center for PEP [19]. Singh performed a retrospective review of PEP at the University of Nebraska in 109 liver transplant patients that received 235 ERCPs [20]. Li et al retrospectively reviewed 48 ERCPs for complications from 48 patients with liver transplant at Zhongnan Hospital in Wuhan, China [21]. Faleschini et al retrospectively reviewed 490 post-liver transplant ERCPs in 142 patients for biliary complications at the University of Udine, Italy [22]. Ramesh et al performed a retrospective review of 210 ERCPs in 210 liver transplant patients for post-ERCP complications at the University of Alabama in Birmingham [23]. Catron et al performed a retrospective review of complications after 66 ERCPs in 43 liver transplant patients at the University of Alabama in Birmingham [24].
Meta-analysis results
Post-ERCP complication rate
Fourteen studies met our inclusion criteria and were included in this analysis [5, 12-24]. These studies include a total of 3,192 ERCPs performed on 1,787 patients. The pooled all-complication rate was 5.2% (95% CI: 0.035 - 0.075) (Fig. 2). Procedural complications analyzed included PEP 3.4% (95% CI: 0.025 - 0.047), bleeding 1.1% (95% CI: 0.006 - 0.020), infections 0.2% (95% CI: 0.025 - 0.047), and cholangitis 0.8% (95% CI: 0.004 - 0.020) (Fig. 3a-d). No cases of periprocedural death were reported.
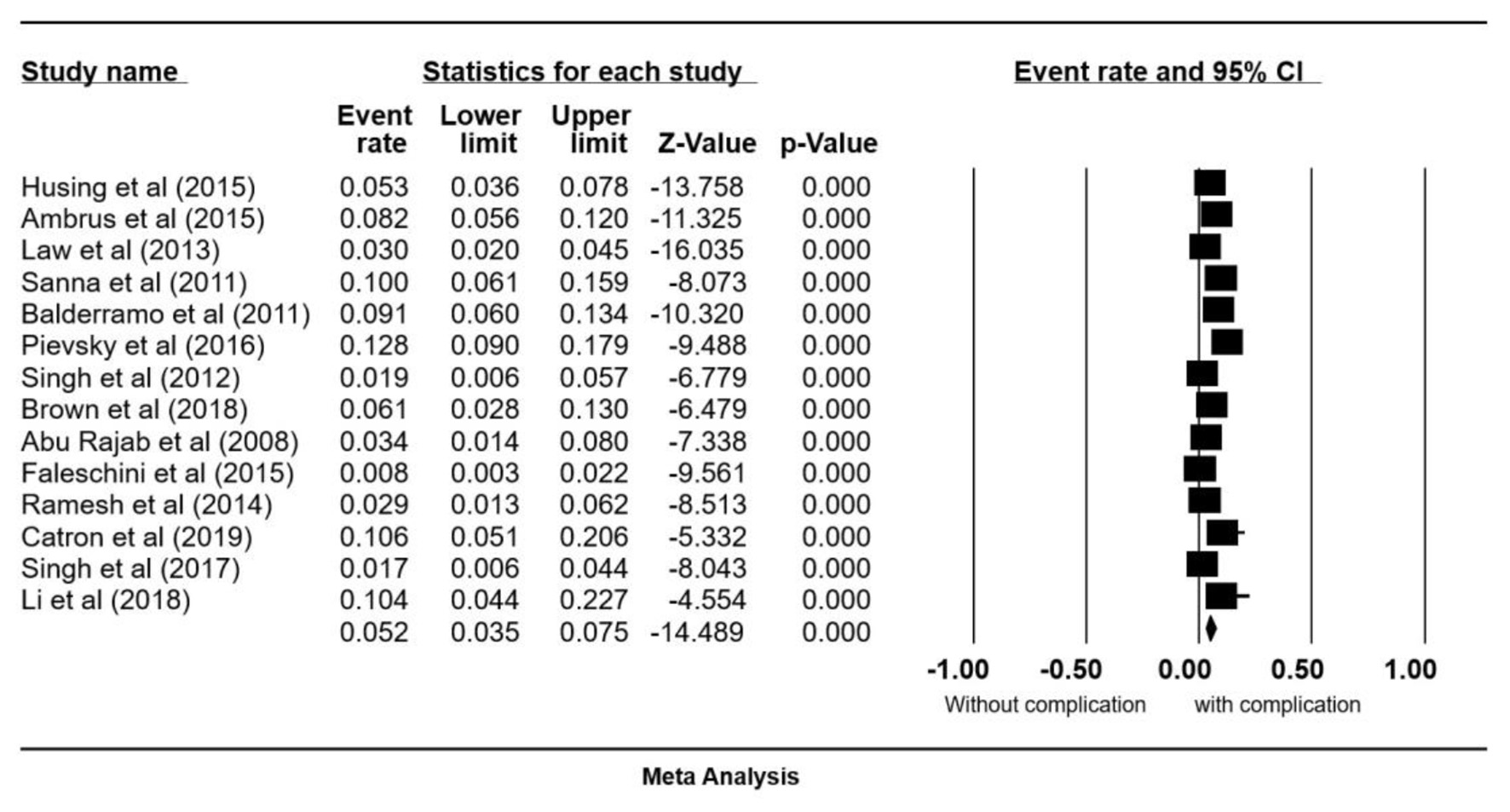 Click for large image | Figure 2. Forrest plot, post-ERCP all-complication rate in liver transplant patients. ERCP: endoscopic retrograde cholangiopancreatography; CI: confidence interval. |
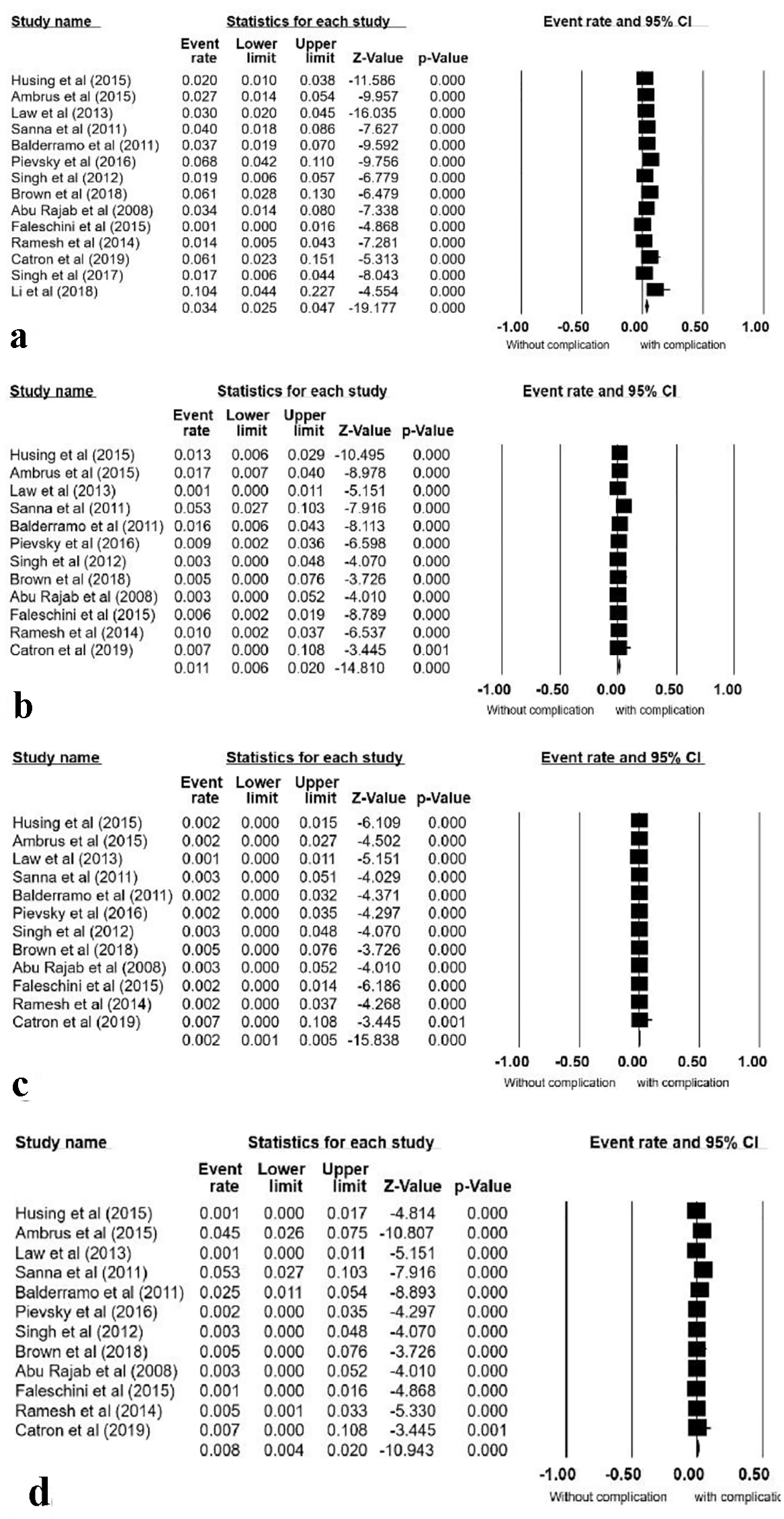 Click for large image | Figure 3. Forrest plots, pooled event rate for post-ERCP pancreatitis (a), bleeding (b), infection (c) and cholangitis (d). ERCP: endoscopic retrograde cholangiopancreatography; CI: confidence interval. |
Evaluation for publication bias
A Funnel plot was generated to evaluate whether patients were more likely to have PEP with liver transplant versus without liver transplant (Fig. 4b). The plot is symmetric and does not suggest the presence of publication bias. Egger’s regression asymmetry testing was also done to demonstrate no evidence of publication bias (P > 0.05).
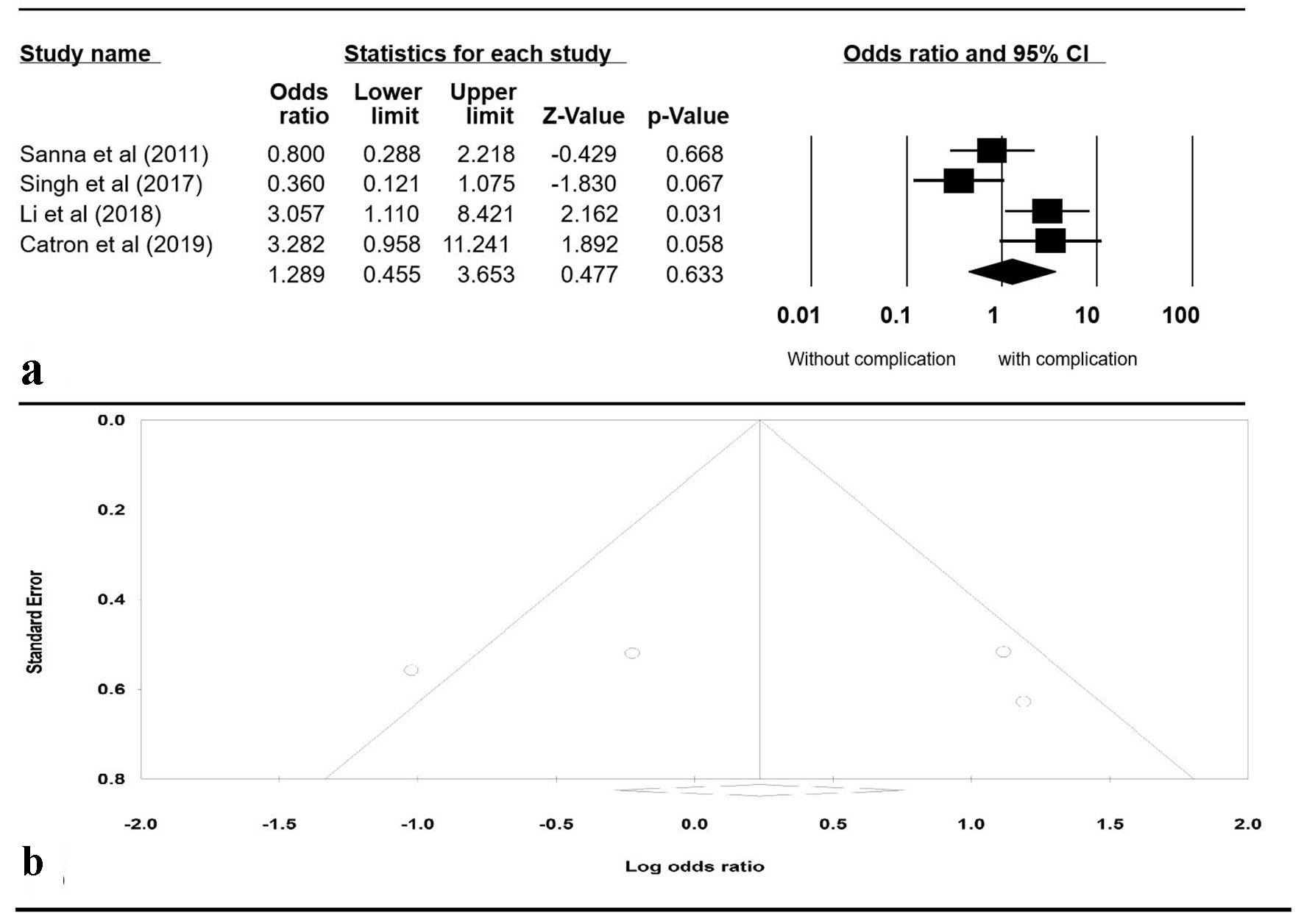 Click for large image | Figure 4. (a) Forrest plot, pooled OR of post-ERCP pancreatitis versus non-transplant patients. (b) Funnel plot of standard error by log OR. OR: odds ratio; ERCP: endoscopic retrograde cholangiopancreatography; CI: confidence interval. |
Sensitivity analysis
To review sensitivity, one study was excluded at a time to observe its individual effect on the pooled OR. The pooled effect estimates from this analysis remained approximately the same. A subgroup analysis including only the studies that evaluated PEP was performed separately. It included four studies: Sanna et al [14], Singh [20], Li et al [21], and Catron et al [24]. The pooled OR for PEP in patients with liver transplant compared to patients without liver transplant was 1.289 (95% CI: 0.455 - 3.653, P = 0.633, I2 = 72.88%) (Fig. 4).
| Discussion | ▴Top |
Post-operative biliary complications remain a noteworthy cause of morbidity and mortality in liver transplant recipients [3]. Some of these complications require surgical revision in some cases; however, most can be managed with a less invasive approach, such as ERCP, which stands as a pivotal tool in managing a plethora of these complications [6-8].
While management of these complications with ERCP has become more common, the literature evaluating the safety of this procedure in liver transplant patients remains scarce and contradicting. Previous studies have suggested that PEP remains the most common complication before and after liver transplant [9]. This was followed by infection, while bleeding was the second most common complication in non-transplant patients [9]. Although it was reported that post-ERCP bleeding is more frequent in liver transplant patients compared to the general population [9], other studies have shown no difference in post-ERCP bleeding between the two populations [15].
In addition to the paucity in safety data, endoscopists could have theoretical concerns of difficult anatomy and cannulation in this patient population. To our knowledge, this is the first meta-analysis aimed to examine the previously published studies on post-ERCP complications in patients with liver transplant.
Our analysis included a total of 14 studies with 1,787 patients undergoing a total of 3,192 ERCPs. The analyzed studies covered a multitude of hospitals and universities in the USA, Europe, and China over a broad time period (as early as 1990 to as recent as 2018). The diverse study population incorporated in this meta-analysis allows for the results to be generalizable across various settings in liver transplantation.
Indications for liver transplant were reported in many of the studies included in our analysis. The three most common indications for liver transplant were hepatitis B/C cirrhosis, alcoholic cirrhosis, and hepatocellular carcinoma [5, 14, 22]. Similarly, various studies of the included cohort reported several indications for ERCP. The three most common indications for post-transplant ERCP, among many, included bile duct strictures, bile leaks, and choledocholithiasis [5, 13-15, 19, 22, 24]. The wide variety of indications for both liver transplant and post-transplant ERCP allow for a more complete perspective of the therapeutic effects of ERCP in multiple populations with variable risks.
Since the first study discussed post-ERCP complications in 1990 by Sanna et al [14], 10 other studies were published to date which specifically analyzed PEP, bleeding, infection, and cholangitis [5, 12, 13, 15-19, 22, 23]. The majority of these studies found the risk of these complications in liver transplant patients to be comparable to historically reported risk [25-27].
A few studies from our meta-analysis, namely Sanna et al [14], Singh [20], Li et al [21], and Catron et al [24], compared the incidence of post-ERCP complications in patients with and without liver transplant. While Sanna et al and Catron et al showed that the incidence of total adverse events was similar amongst both populations, Singh concluded that liver transplant patients are at a lower overall risk of developing PEP compared to the general population. In contrast, Catron et al found a non-significant trend toward higher incidence of PEP in patients with liver transplant compared to those without liver transplant. Li et al replicated Catron et al findings, although, with statistical significance and they further classified the risk by discerning specific interventions, such as fistulotomy and pancreatic deep wire cannulation which could potentially increase the risk of PEP.
The results of this meta-analysis suggest that post-ERCP complication rates in liver transplant patients are comparable to the general population undergoing ERCP. This could likely be explained by merely a lack of difference in complication rates between both groups. Another school of thought went further to suggest that a multitude of factors including the use of immunosuppressants in transplant patients and the advancements made in this field over the past decade might offset any potential increase in complication rates. The use of immunosuppressive agents in transplant recipients may be protective against any possible inflammatory response implicit in complications such as pancreatitis [17]. Furthermore, transplant patients may also be managed more acutely at higher level centers where procedures are performed by higher volume endoscopists, providing early recognition and prompt management hindering the need for repeat procedures and subsequently further perioperative complications.
A key limitation of our meta-analysis is the inconsistency in controlling for patient- and technique-related characteristics among the studies included. For example, most studies failed to control for previous history of biliary complications, the cause of cirrhosis, and the expertise of the endoscopist. Moreover, presence of relevant peri-operative complications such as prolonged ischemia time and biliary leak, as well as primary sclerosing cholangitis recurrence in transplanted graft were not characterized as possible factors attributing to selection bias in the analyzed studies. Additionally, only four studies of the completed cohort compared post-ERCP complications between the general population and transplant patients. Although significant heterogeneity was observed in our sensitivity analysis, the limited number of analyzed studies precluded us from performing subsequent sub-group analysis or a meta-regression based on the design, location, and quality of the studies.
In conclusion, our results suggest that post-ERCP complication rates in liver transplant patients are comparable to those in the general population, and hence, peri-procedural evaluation and management may be continued as per the current standards of care without pre-conceived notions about possible increased risk of complications secondary to liver transplantation status that might lead to unnecessary delays or referrals to higher volume centers. Future prospective randomized research is needed to confirm these results.
Acknowledgments
The preliminary data of this work were presented at the American College of Gastroenterology (ACG) 2019 conference (October 25 - 30, San Antonio, TX).
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Not applicable.
Author Contributions
MA, SJ, PT and MS contributed conception and design of the study and provided administrative support; LA, MA, AK and VP collected and assembled data; LA, MA, AK, AH, SK and VP analyzed and interpreted the data; LA and MA wrote the first draft of the manuscript. All authors contributed to paper writing, manuscript revision and approved the submitted version.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Global observatory on donation and transplantion. GODT. http://www.transplant-observatory.org. Accessed July 6, 2019.
- Health Resources and Services Administration, U.S. Department of Health and Human Services. Organ Procurement & Transplantion Network. https://optn.transplant.hrsa.gov/. Accessed July 6, 2019.
- Watt KD, Pedersen RA, Kremers WK, Heimbach JK, Charlton MR. Evolution of causes and risk factors for mortality post-liver transplant: results of the NIDDK long-term follow-up study. Am J Transplant. 2010;10(6):1420-1427.
doi pubmed - Kobryn K, Koziel S, Porecka M, Kobryn K, Holowko W, Patkowski W, Zieniewicz K, et al. Endoscopic treatment of early biliary complications in liver transplant recipients. Ann Transplant. 2015;20:741-746.
doi pubmed - Husing A, Cicinnati VR, Maschmeier M, Schmidt HH, Wolters HH, Beckebaum S, Kabar I. Complications after endoscopic sphincterotomy in liver transplant recipients: A retrospective single-centre study. Arab J Gastroenterol. 2015;16(2):46-49.
doi pubmed - Macfarlane B, Davidson B, Dooley JS, Dawson K, Osborne MJ, Rolles K, Burroughs AK. Endoscopic retrograde cholangiography in the diagnosis and endoscopic management of biliary complications after liver transplantation. Eur J Gastroenterol Hepatol. 1996;8(10):1003-1006.
doi pubmed - Bourgeois N, Deviere J, Yeaton P, Bourgeois F, Adler M, Van De Stadt J, Gelin M, et al. Diagnostic and therapeutic endoscopic retrograde cholangiography after liver transplantation. Gastrointest Endosc. 1995;42(6):527-534.
doi - Roos FJM, Poley JW, Polak WG, Metselaar HJ. Biliary complications after liver transplantation; recent developments in etiology, diagnosis and endoscopic treatment. Best Pract Res Clin Gastroenterol. 2017;31(2):227-235.
doi pubmed - Sanna C, Saracco GM, Reggio D, Moro F, Ricchiuti A, Strignano P, Mirabella S, et al. Endoscopic retrograde cholangiopancreatography in patients with biliary complications after orthotopic liver transplantation: outcomes and complications. Transplant Proc. 2009;41(4):1319-1321.
doi pubmed - Wells GA, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. 2012.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188.
doi - Ambrus RB, Svendsen LB, Hillingso JG, Hansen ML, Achiam MP. Post-endoscopic retrograde cholangiopancreaticography complications in liver transplanted patients, a single-center experience. Scand J Surg. 2015;104(2):86-91.
doi pubmed - Law R, Leal C, Dayyeh BA, Leise MD, Balderramo D, Baron TH, Cardenas A. Role of immunosuppression in post-endoscopic retrograde cholangiopancreatography pancreatitis after liver transplantation: a retrospective analysis. Liver Transpl. 2013;19(12):1354-1360.
doi pubmed - Sanna C, Giordanino C, Giono I, Barletti C, Ferrari A, Recchia S, Reggio D, et al. Safety and efficacy of endoscopic retrograde cholangiopancreatography in patients with post-liver transplant biliary complications: results of a cohort study with long-term follow-up. Gut Liver. 2011;5(3):328-334.
doi pubmed - Balderramo D, Bordas JM, Sendino O, Abraldes JG, Navasa M, Llach J, Cardenas A. Complications after ERCP in liver transplant recipients. Gastrointest Endosc. 2011;74(2):285-294.
doi pubmed - Pievsky D, Arrigo RJ, Okoronkwo N, et al. Complications of ERCP in post-liver transplant patients. Gastrointestinal Endoscopy. 2016;83(5):AB201-AB202.
doi - Singh S, Bhat I, Chakraborty S, et al. Post-ERCP pancreatitis in liver transplant patients: single center experience. Gastrointestinal Endoscopy. 2012;75(4):AB311.
doi - Brown NG, Harika G, Bari K, et al. A single academic center evaluation of tacrolimus and post-ERCP pancreatitis. In: Philadelphia, PA: American College of Gastroenterology; 2018.
doi - Abu Rajab MA, Gan SI. Are post-orthotopic liver transplant patients at higher risk for post-ERCP-pancreatitis? Gastroenterology. 2008;134(4):A805.
doi - Singh S. Risk of post-ERCP pancreatitis in liver transplant patients: single center experience. Theses & Dissertations. 2017. https://digitalcommons.unmc.edu/etd/240.
- Li GZ, Wang F, Fang J, Zha HL, Zhao Q. Risk factors for post-endoscopic retrograde cholangiopancreatography pancreatitis: evidence from 1786 cases. Med Sci Monit. 2018;24:8544-8552.
doi pubmed - Faleschini G, Vadala di Prampero SF, Bulajic M, Baccarani U, Toniutto P, Panic N, Zoratti LM, et al. Predictors of endoscopic treatment outcome in the management of biliary complications after orthotopic liver transplantation. Eur J Gastroenterol Hepatol. 2015;27(2):150-154.
doi pubmed - Ramesh J, Reddy N, Kim H, Monkemuller K, Varadarajulu S, McGuire B, DuBay D, et al. Safety and yield of diagnostic ERCP in liver transplant patients with abnormal liver function tests. Diagn Ther Endosc. 2014;2014:314927.
doi pubmed - Catron TD, Shipley L, Johnson D, et al. Differences in post-ERCP adverse event rates between post-liver transplant patients and patients with native hepatobiliary anatomy. Gastrointestinal Endoscopy. 2019;89(6):AB211-AB212.
doi - Talukdar R. Complications of ERCP. Best Pract Res Clin Gastroenterol. 2016;30(5):793-805.
doi pubmed - Andriulli A, Loperfido S, Napolitano G, Niro G, Valvano MR, Spirito F, Pilotto A, et al. Incidence rates of post-ERCP complications: a systematic survey of prospective studies. Am J Gastroenterol. 2007;102(8):1781-1788.
doi pubmed - Cotton PB, Lehman G, Vennes J, Geenen JE, Russell RC, Meyers WC, Liguory C, et al. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37(3):383-393.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


