| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website http://www.gastrores.org |
Case Report
Volume 13, Number 2, April 2020, pages 81-84
Reactivation of Epstein-Barr Virus Hepatitis in T/Natural Killer (NK) Cells Mimicking Liver T/NK-Cell Lymphoma
Sara Kwonga, Xiangping Lub, Xiuli Liuc, Jinping Laid, e
aDepartment of Pathology and Laboratory Medicine, University of California, Davis-School of Medicine, Sacramento, CA, USA
bDepartment of Pathology and Laboratory Medicine, Kaiser Permanente San Leandro Medical Center, San Leandro, CA, USA
cDepartment of Pathology and Laboratory Medicine, University of Florida College of Medicine, Gainesville, FL, USA
dDepartment of Pathology and Laboratory Medicine, Kaiser Permanente Sacramento Medical Center, Sacramento, CA, USA
eCorresponding Author: Jinping Lai, Department of Pathology and Laboratory Medicine, Kaiser Permanente Sacramento Medical Center, 2025 Morse Ave., Sacramento, CA, USA
Manuscript submitted March 31, 2020, accepted April 6, 2020
Short title: Reactivation of EBV Hepatitis
doi: https://doi.org/10.14740/gr1283
| Abstract | ▴Top |
Diagnosis of Epstein-Barr virus (EBV)-associated hepatitis, chronic active EBV infection, and EBV-associated lymphoproliferative diseases, is always challenging due to the overlapping symptoms and lack of diagnostic criteria. We report such a case of a 40-year-old man with unremarkable past medical history. He presented with fever of unknown origin for 1 month with jaundice for 2 days. Physical exams were unremarkable with body temperature at 98.6 °F. His liver function tests were elevated with alanine transaminase (ALT) 559 U/L, aspartate transaminase (AST) 892 U/L, alkaline phosphatase 319 U/L and total bilirubin 4.4 mg/dL. Computed tomography of his chest, abdomen and pelvis did not show lymphadenopathy or hepatosplenomegaly. A liver biopsy showed moderately acute hepatitis with hemophagocytosis, positive Epstein-Barr virus encoding RNA (EBER) in situ hybridization in CD3 and CD4-positive T cells and CD56-positive natural killer (NK) cells. CD20 was negative. The pathology diagnosis was consistent with reactivation of EBV hepatitis but NK-cell lymphoma needs to be excluded. Steatohepatitis with mild activity was present. His blood EBV DNA was 846,000 copies/mL and continued to increase to 2,000,000 copies/mL. Flow cytometric analysis of his bone marrow revealed an increased NK-cell activity but no T/NK-cell lymphoma was identified. Initial treatment with rituxan, etoposide and/or ruxolitinib/acyclovir failed or only had limited effect. However, subsequent valganciclovir greatly improved his conditions. In his 3 months follow-up, the patient was doing well with almost normal liver function tests except mildly elevated ALT (95 U/L) that was due to mild steatohepatitis. EBV DNA PCR was 2,009 copies/mL. To the best of our knowledge, this is the first documented case with reactivation of EBV hepatitis mimicking NK-cell lymphoma in the English literature. With appropriate anti-EBV viral treatments, the patient eventually became asymptomatic and was able to return to his routine life.
Keywords: Chronic active EBV infection; Hepatitis; T/NK-cell lymphoma; EBV-associated hemophagocytic lymphohistiocytosis; Steatohepatitis
| Introduction | ▴Top |
Approximately 90% of the general population is seropositive for Epstein-Barr virus (EBV) by early adulthood [1]. Although majority of the EBV infections manifest as infectious mononucleosis and usually result in spontaneous resolutions, on rare occasions, they may result in more serious clinical conditions, such as liver failure [2], chronic active EBV infections (CAEBV) and lymphoproliferative disorders, including hemophagocytic lymphohistiocytosis (HLH) and extra-nodal natural killer (NK)/T-cell lymphomas [3].
Although many of the primary or secondary hepatic lymphomas demonstrate more obvious histologic findings, there are some lymphomas showing very subtle histologic changes mimicking inflammatory/infectious process. Therefore, it is extremely important for the liver pathologists to recognize the benign and malignant mimickers and be familiar with laboratory findings, clinical symptoms and past medical history [4]. Through PubMed search, reactivation of EBV hepatitis involving NK cells is rarely reported. Cases of EBV reactivation with acute episode of hepatitis mimicking NK-cell lymphoma in patients with steatohepatitis have not been previously reported in the English literature. We report such a case and the diagnostic and treatment challenges are discussed.
| Case Report | ▴Top |
A 40-year-old man with unremarkable past medical history except occasional common colds presented with mild cytopenia and fever of unknown origin that was fluctuating between 102 and 104 °F for approximately 1 month and jaundice for 2 days prior to the presentation.
On admission, the laboratory values were notable for elevated liver function tests (alanine transaminase (ALT) 559 U/L, aspartate transaminase (AST) 892 U/L, alkaline phosphatase 319 U/L and total bilirubin 4.4 mg/dL), hypertriglyceridemia (fasting triglycerides 600 mg/dL), increased ferritin (3,000 ng/mL) and mild cytopenia (hemoglobin 11 g/dL, platelet 90 × 103/µL, and white blood cell (WBC) in normal range). The physical examination was unremarkable with normal body temperate at 98.6 °F. Computed tomography of his chest, abdomen and pelvis showed no lymphadenopathy or hepatosplenomegaly. A liver biopsy was performed and submitted to pathology.
The main histologic findings included portal inflammation with interface activity, lobular inflammation with spotty necrosis and focal confluent necrosis admixed with some lobular inflammation caused by steatohepatitis. The lobular inflammation appears more prominent than a conventional steatohepatitis. Large droplet fat was present in about 25% of the hepatocytes and ballooning hepatocytes were occasionally seen. Sinusoidal lymphocytosis with some individual atypical lymphocytes and hemophagocytosis were present (Fig. 1). The atypical lymphoid cells were positive for CD3, CD56, CD4, rarely positive for CD8, and negative for CD20. Epstein-Barr virus encoding RNA (EBER) in situ hybridization with appropriate control was positive in the individual CD3-positive T cells and CD56-positive NK cells (Fig. 2). The EBER-positive T/NK cells were present in the portal chronic inflammation and interface hepatitis and lobular inflammation suggesting EBV-associated with hepatitis present in the background of mild steatohepatitis. Trichrome stain highlights minimal zone 3 perisinusoidal fibrosis that was not seen on hematoxylin-eosin (H&E) stain. Due to a short history of possible EBV infection in 2017, the pathologic diagnosis was consistent with EBV-associated moderately acute hepatitis consistent with chronic reactivation of EBV hepatitis and steatohepatitis with mild activity (non-alcoholic fatty liver disease (NALFD) activity score (NAS) score 4/8) with focal perisinusoidal fibrosis only seen on trichrome stain (fibrosis stage 1a of 4). Although no significant clusters or piling up of atypical T/NK cells were identified, a possibility of T/NK-cell lymphoma could not be excluded on the liver biopsy.
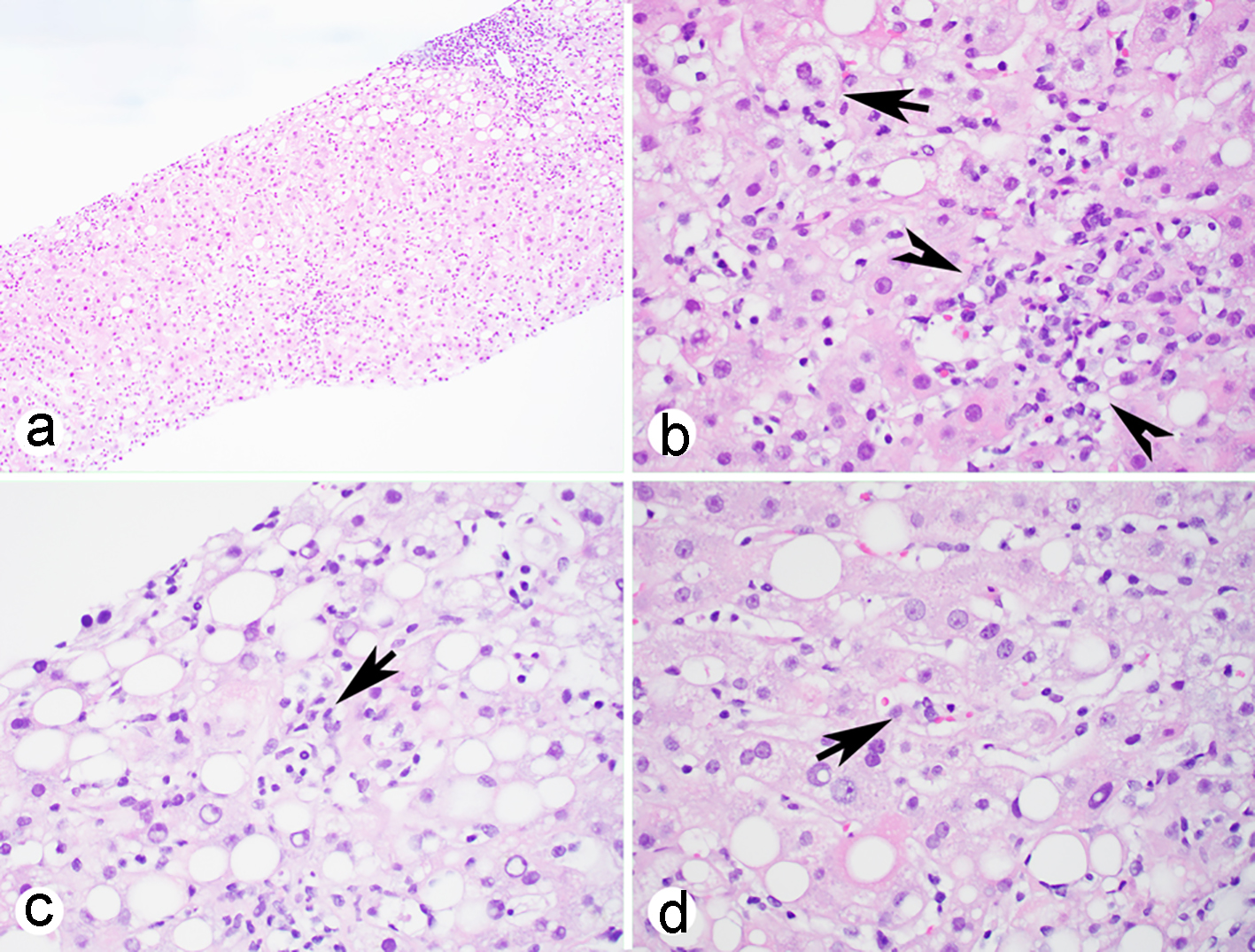 Click for large image | Figure 1. Liver biopsy shows acute hepatitis with portal inflammation (a), lobular confluent necrosis (b, arrow heads), mild steatosis and occasional ballooning hepatocytes (b, arrow) and atypical lymphoid cells in the sinusoids (c, arrow) with phagocytosis (d, arrow) (H&E stain: a, × 100, b-d, × 400). H&E: hematoxylin-eosin. |
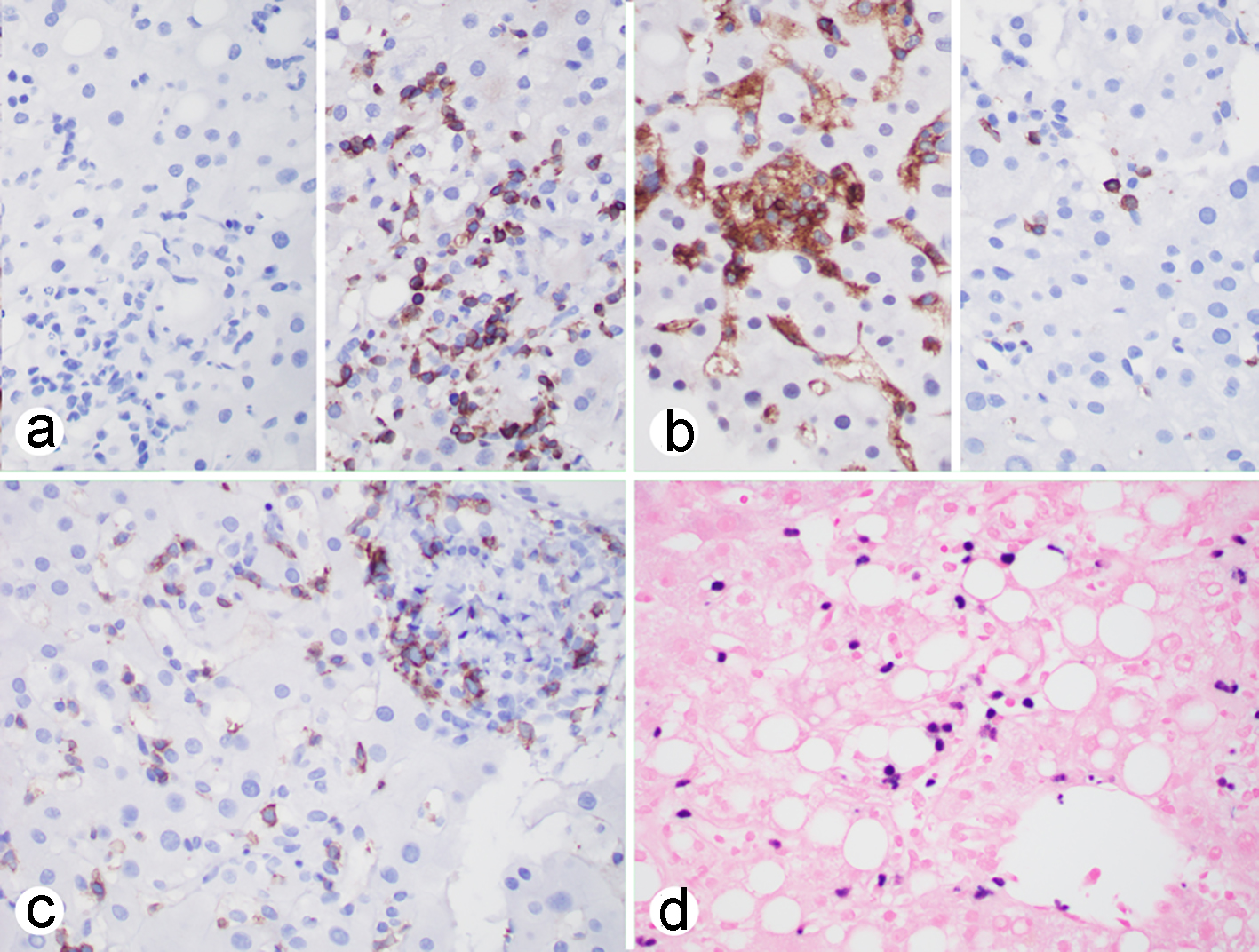 Click for large image | Figure 2. EBV-associated hepatitis: (a-c) immunohistochemistry showing that the atypical lymphoid cells are negative for CD20 (a, left), positive for CD3 (a, right), CD4 (b, left), rarely positive for CD8 (b, right), positive for CD56 (c); (d) EBER in situ hybridization showing that individual lymphoid cells are positive. (a-d, × 400). EBV: Epstein-Barr virus; EBER: Epstein-Barr virus encoding RNA. |
The patient was deteriorating with worsening fever, increased weakness and abdominal pain and he received rituxan treatment for CAEBV of NK-cell type. Bone marrow biopsy was performed and showed EBER-positive lymphocytes and hemophagocytosis. The flow cytometric analysis showed an increased NK-cell activity and no NK-cell lymphoma was identified. The patient was initially improving on weekly treatment of etoposidep; however, he was declining after 2 weeks. The EBV viral load was continuously increasing from 846,000 to 2,000,000 copies/mL. Additional treatment with ruxolitinib (Jakafi) and acyclovir was initiated with no improvement. Eventually, the patient was treated with valganciclovir and started to show clinical improvement. Patient eventually became asymptomatic and returned to his routine lifestyle. In his 3 months follow-up, he was doing well and his liver function tests were almost normal except mildly elevated ALT (95 U/L) that was due to existence of mild steatohepatitis. His complete blood cell (CBC) test was in the normal range and EBV viral load was 2,009 copies/mL by EBV DNA PCR.
| Discussion | ▴Top |
It is important to distinguish self-limited EBV-associated hepatitis from other EBV-associated hematological diseases, such as CAEBV or acute fulminant EBV-associated hemophagocytic lymphohistiocytosis (EBV-HLH) and other EBV-associated lymphoproliferative diseases. It is often quite challenging due to the lack of specificity and sensitivity of the current diagnostic methodologies, and rather it is the combination of the clinical, histologic findings and the ancillary studies that will yield the correct diagnosis [3, 4].
The most common prototype of EBV infection is infectious mononucleosis (IM). Often, the symptoms are nonspecific, though approximately half of them have hepatitis and 5-10% patients may develop jaundice [1]. The most characteristic histologic feature of the EBV-associated hepatitis is the “string of beads” pattern demonstrated by the single file of the lymphocytes infiltrating the hepatic sinusoids. The overall lobular architecture remains intact, though focal spotty necrosis or steatosis may be seen as in our case. Often, the portal tract will also show lymphocytic expansion, predominantly consisting of cytotoxic (CD8+) T lymphocytes and less frequently intermixed with NK cells or B cells.
A primary EBV infection may lead to a rare entity, CAEBV. The defined criteria for CAEBV include persistent IM-like symptoms for greater than 3 months, increased EBV DNA (> 102.5 copies/mg) in peripheral blood, histological evidence of organ disease and EBV DNA or viral protein in affected tissue [4, 5]. Interestingly, majority of the Asian cases show monoclonal proliferation of CD4+ T cells or NK cells and majority of the US cases have shown to have proliferation of the B cells [3]. The histologic findings are nonspecific and may mimic autoimmune hepatitis or other viral induced hepatitis, including lobular hepatitis and portal tract expansion with plasma cell infiltrations [3]. Bridging fibrosis, hepatic cords disarray and interface hepatitis may also be present. EBER positivity in tissue and high EBV DNA titers in the peripheral or whole blood should prompt the differential diagnosis of CAEBV. Early recognition of CAEBV is of paramount importance due to the poor prognosis, especially in the adult-onset cases, and the potential progression to the fatal complications, such as hemophagocytic syndrome.
For the diagnosis of HLH, five of the eight diagnostic criteria must be met, and they include fever (> 38.5 °C for longer than 7 days), splenomegaly (spleen palpable > 3 cm below costal margin), cytopenia involving more than two cell lines (hemoglobin < 9 g/dL, absolute neutrophil count < 100/µL, platelets < 100,000/µL), hypertriglyceridemia (fasting triglycerides > 177 mg/dL or > 3 standard deviations more than normal value for age), low or absent NK-cell activity, increased ferritin (> 500 ng/mL), increased soluble interleukin-2 (CD25) and hemophagocytosis [6]. Although hemophagocytosis can be seen in HLH biopsies, it is a nonspecific finding, which can also be seen in inflammatory/infectious processes. Since HLH is a rare disorder, there are a limited number of histopathologic studies. One post-mortem examination of the livers from HLH patients has shown lymphocytic infiltration of the portal tracts, mimicking chronic hepatitis. Contrary to the EBV-associated hepatitis, as in our case, most of these lymphocytes are usually CD4+ helper T cells and CD56+ NK cells and very few are CD8+ cytotoxic T cells. Moreover, there are some histiocytes involved in the portal tracts and sinusoids exhibiting hemophagocytosis. Interestingly, the histologic findings of the hemophagocytosis may attenuate or even disappear with the treatment of corticosteroids or cytostatic drugs [7, 8]. Therefore, it is vitally important to incorporate clinical findings and ancillary studies to formulate the diagnosis of HLH. Our case also had steatohepatitis that could be associated with the increase of triglyceride level and did not meet the criteria for diagnosis of HLH, although hemophagocytosis was present in the liver biopsy. Majority of CD4 T cells and NK cells with rare CD8 T cells present in our liver biopsy favor CAEBV over HLH. However, during the disease course, CAEBV can lead to two lethal conditions: HLH and chemotherapy-resistant lymphoma. Clinical follow-up is recommended and it may be necessary to start treatment before these conditions develop in some cases [9].
Primary hepatic lymphomas (PHLs) are rare and approximately 0.4% of extra-nodal lymphomas are PHL, with diffuse large B-cell lymphoma (DLBCL) being the most common type. Although many do exhibit partial or complete effacement of the hepatic architecture, necrosis and increased number of atypical mitosis, a small subset of PHL may demonstrate more subtle histologic changes, such as portal and/or sinusoidal infiltration mimicking a benign process. NK-cell lymphoma seen in the liver is frequently a part of systemic disease and the NK lymphoma histologically shows the atypical NK cells piling up in the sinusoids that was no seen in our case and further flow cytometry of the bone marrow showed increased NK-cell activity but no NK-cell lymphoma was identified. Therefore, it is important for a liver pathologist to be mindful and be familiar with clinical symptoms, laboratory results (EBV viral load/EBV DNA copy numbers, EBV antigens) and past medical history (transplant and/or immunodeficiency), and initiate hematological workup accordingly, including immunohistochemistry, flow cytometry, molecular testing and other ancillary tests [4].
In conclusion, we report a rare case of acute episode of reactivation of EBV hepatitis mimicking NK-cell lymphoma. Though histologic examination of the tissue may assist in diagnosis of EBV-associated hepatitis or CAEBV, as well as hemophagocytic syndrome or other lymphoproliferative diseases, its utility is quite limited due to the nonspecific features and a detailed clinical history and ancillary tests are critical in differentiating EBV-associated lymphoproliferative disorders from reactivation of EBV-associated hepatitis. The liver pathologist should be mindful and be familiar with clinical symptoms, laboratory results (EBV viral load) and past medical history, and initiate hematological workup accordingly. With appropriate anti-EBV treatments, some patients can become asymptomatic and be able to return to a routine life.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
All authors have approved this article and declare that they have no conflict of interest.
Informed Consent
Not applicable.
Author Contributions
SK wrote the manuscript; XL and XL reviewed the manuscript; JL made the diagnosis, collected and analyzed the data, wrote and finalized the manuscript.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
| References | ▴Top |
- Petrova M, Kamburov V. Epstein-Barr virus: silent companion or causative agent of chronic liver disease? World J Gastroenterol. 2010;16(33):4130-4134.
doi pubmed - Zhang W, Chen B, Chen Y, Chamberland R, Fider-Whyte A, Craig J, Varma C, et al. Epstein-barr virus-associated acute liver failure present in a 67-year-old immunocompetent female. Gastroenterology Res. 2016;9(4-5):74-78.
doi pubmed - Fernandez-Pol S, Silva O, Natkunam Y. Defining the elusive boundaries of chronic active Epstein-Barr virus infection. Haematologica. 2018;103(6):924-927.
doi pubmed - Choi WT, Gill RM. Hepatic lymphoma diagnosis. Surg Pathol Clin. 2018;11(2):389-402.
doi pubmed - Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. (4th ed.) IARC, Lyon, 2017.
- Hayden A, Lin M, Park S, Pudek M, Schneider M, Jordan MB, Mattman A, et al. Soluble interleukin-2 receptor is a sensitive diagnostic test in adult HLH. Blood Adv. 2017;1(26):2529-2534.
doi pubmed - Ost A, Nilsson-Ardnor S, Henter JI. Autopsy findings in 27 children with haemophagocytic lymphohistiocytosis. Histopathology. 1998;32(4):310-316.
doi pubmed - Pinto-Patarroyo GP, Rytting ME, Vierling JM, Suarez-Almazor ME. Haemophagocytic lymphohistiocytosis presenting as liver failure following Epstein-Barr and prior hepatitis A infections. BMJ Case Rep. 2013;2013.
doi pubmed - Arai A. Advances in the Study of Chronic Active Epstein-Barr Virus Infection: Clinical Features Under the 2016 WHO Classification and Mechanisms of Development. Front Pediatr. 2019;7:14.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


