| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website http://www.gastrores.org |
Original Article
Volume 11, Number 1, February 2018, pages 25-30
Metastatic Renal Cell Carcinoma as Solitary Subcentimeter Polypoid Gastric Mucosal Lesions: Clinicopathologic Analysis of Five Cases
Amanda Hemmericha, e, Mohanad Shaara, e, Rebecca Burbridgeb, Cynthia D. Guya, Shannon J. McCalla, Diana M. Cardonaa, Xuchen Zhangc, Jinping Laid, Xuefeng Zhanga, f
aDepartment of Pathology, Duke University Medical Center, Durham, NC, USA
bDivision of Gastroenterology, Duke University Medical Center, Durham, NC, USA
cDepartment of Pathology, Yale School of Medicine, New Haven, CT, USA
dDepartment of Pathology, University of Florida College of Medicine, Gainesville, FL, USA
eThese authors contributed equally to this work
fCorresponding Author: Xuefeng Zhang, Department of Pathology, Duke University Medical Center, Box 3712, DUMC, Durham, NC 27710, USA
Manuscript submitted January 3, 2018, accepted January 23, 2018
Short title: Metastatic RCC as a Small Gastric Polyp
doi: https://doi.org/10.14740/gr952w
| Abstract | ▴Top |
Background: The stomach is an uncommon site for metastatic carcinoma. Approximately 6% of renal cell carcinomas (RCCs) may metastasize to the stomach. The majority of the reported metastatic RCCs in the stomach presented as large masses or ulcers greater than a centimeter in size. It is very rare to encounter metastatic RCC as a solitary small polypoid gastric mucosal lesion.
Methods: In this study, we collected surgical pathology cases of gastric metastasis from RCC that measured 1.0 cm or less at the time of endoscopy. The clinicopathological characteristics were analyzed.
Results: Five patients with subcentimeter metastatic RCC involving the gastric mucosa were identified. The clinical presentation for upper endoscopic examination was non-specific. Two of the five patients did not have a known history of RCC. In the three patients with a previous history of RCC, the interval from primary RCC diagnosis to the detection of gastric mucosal metastasis was 5, 6, and 10 years, respectively. Endoscopically, all the lesions were solitary, ranging in size from 0.4 to 1 cm. Histologically, all five cases were of the clear cell type consisting of a bland clear cell proliferation within the lamina propria. Although the tumor cells were relatively bland, the presence of clear cytoplasm, nuclear membrane irregularity, occasional enlarged hyperchromatic atypical nuclei, and destructive growth in the center of the lesion should promote immunohistochemical workup. Immunohistochemically, the RCC cells exhibited at least patchy immunoreactivity for cytokeratin and RCC markers. In two cases, there were many CD68 positive foamy histiocytes intermingled with the tumor cells.
Conclusion: Metastatic RCC can rarely present as subcentimeter polypoid gastric mucosal lesions. The remote or unknown history of RCC, the non-specific endoscopic appearance, and the bland histological features may lead to a potential diagnostic pitfall. It is of importance to raise the awareness of such an unusual presentation of metastatic RCC in the stomach and to include metastatic RCC in the differential diagnosis for gastric mucosal polyps with clear cell morphology.
Keywords: Gastric polyp; Renal cell carcinoma; Metastasis; Gastric metastasis
| Introduction | ▴Top |
The stomach is an uncommon site for metastatic carcinoma of any given primary. In several previously published large autopsy series (from 1952 to 1990), the stomach has been reported as a metastatic site in 0.2-0.7% of cases [1]. In 2001, Oda et al reported an autopsy series in the Japanese population; metastatic disease to the stomach was present in 5.4% of the patients with solid malignant tumor [2]. In this report, 6.2% of renal cell carcinomas (RCCs) metastasized to the stomach [2]. Namikawa and Hanazaki performed an extensive literature review which demonstrated that breast, lung, and esophageal cancer were the top three primaries to metastasize to the stomach [3]. In that study, RCC was the fourth most common metastatic carcinoma involving the stomach, although the total number of cases was smaller (26 of 341 gastric metastasis cases, 7.6%) [3].
Though most of the gastric metastasis cases were identified in autopsy studies, a subset of reported patients were diagnosed endoscopically. Many of these patients present with very non-specific symptoms such as dysphagia, gastrointestinal bleeding, anemia, dyspepsia, and epigastric pain [1, 2, 4]. In contrast to the multifocal metastasis commonly seen in other organs, the gastric metastasis presents as a single lesion at the time of endoscopy in more than half of the reported cases [1, 2, 4]. Metastatic tumors in the stomach typically exhibit features resembling submucosal tumors or primary gastric carcinoma with deep ulceration [2]. For endoscopists, correctly identifying metastatic disease from a primary gastric lesion is incredibly difficult given there is no definitive endoscopic characteristics to differentiate the two [2]. In addition, the time interval between the diagnoses of the primary tumor and metastatic disease in the stomach has a wide range from 16 to 78 months. RCC is found to have one of the longest intervals between primary diagnosis and metastatic disease with a median interval of 6.5 years [1-5]. Such long intervals and sometimes neglected oncologic history may decrease the clinical suspicion for metastatic disease, and therefore make endoscopic diagnosis of gastric metastasis of RCC even more challenging.
The majority of the reported metastatic RCCs in the stomach present as a large mass or ulcer greater than a centimeter in size [3, 5]. There are only four reported cases with metastatic RCC presenting as small, subcentimeter gastric mucosal lesions [6-9]. It is possible that small foci of gastric metastasis are under-recognized endoscopically and/or histologically, because metastatic RCC may not be in the differential diagnosis for such small lesions, even in patients with a known history of RCC. With the improved endoscopic imaging technologies, it is expected that smaller polypoid gastric metastasis cases will be detected. In this study, we retrospectively analyzed the characteristics of subcentimeter gastric mucosal metastasis from RCC. The study was aimed to raise awareness among pathologists and endoscopists of the existence of small polypoid gastric metastases so that potential diagnostic pitfalls will be recognized and avoided.
| Methods | ▴Top |
The study was approved by the Institutional Review Boards. Surgical pathology specimens of gastric metastasis from RCC that measured 1.0 cm or less at the time of endoscopy were collected from Duke University, University of Florida College of Medicine, and Yale University. Autopsy cases were excluded. The medical records, endoscopy reports and pathology slides were reviewed. Clinical information, endoscopic characteristics, and pathological features were recorded and analyzed.
| Results | ▴Top |
Five patients with subcentimeter metastatic RCC involving the stomach were identified, which included four men and one woman. Mean age at the time of diagnosis of metastatic stomach disease was 71 years old (range 58 - 84 years). Anemia, melena, and gastroesophageal reflux disease were the common indications for upper endoscopy examination. One patient presented with upper gastrointestinal bleeding. Two of the five patients did not have a known history of RCC. In the three patients with previous history of RCC, the interval from primary RCC diagnosis to the detection of gastric mucosal metastasis was 5, 6, and 10 years, respectively. The history of RCC was provided by clinicians in two of these three patients. Three of the five patients had metastatic disease elsewhere in addition to the gastric mucosa, detected either prior to or after the diagnosis of gastric metastasis. The clinicopathologic features are summarized in Table 1 along with the four case reports mentioned above [6-9].
 Click to view | Table 1. Clinicopathologic Characteristics of Metastatic Renal Cell Carcinoma as Subcentimeter Polypoid Gastric Mucosal Lesions |
All the lesions were solitary. The mean size of metastatic lesions on endoscopy was 0.8 cm, ranging from 0.4 to 1.0 cm. One lesion (1.0 cm) resembled a friable mass, whereas the remainder four lesions were endoscopically sessile polyps/nodules. Representative endoscopic images are shown in Figure 1. Histologically, all five cases of RCC in our series were of the clear cell type. The lesions all demonstrated a bland clear cell proliferation within the lamina propria. When the interface between the carcinoma and benign gastric mucosa was biopsied, the clear cells infiltrated among gastric glands without gland destruction (Fig. 2a, b, c). The lesions lacked a rich vascular background. The individual tumor cells showed cytoplasmic vacuoles, small nuclei, nuclear membrane irregularity, and occasional small pinpoint nucleoli. There was only mild nuclear pleomorphism, with occasional tumor cells showing enlarged hyperchromatic nuclei. The nuclear grade was Furhman grade 1 in all the cases. Immunohistochemically, the RCC cells exhibited at least patchy immunoreactivity for cytokeratin and RCC markers, such as PAX8 and RCC, in all the cases (Fig. 2d, e, f). In two cases, there were many foamy histiocytes intermingled with the tumor cells, which were highlighted with immunohistochemistry for CD68 (Fig. 3).
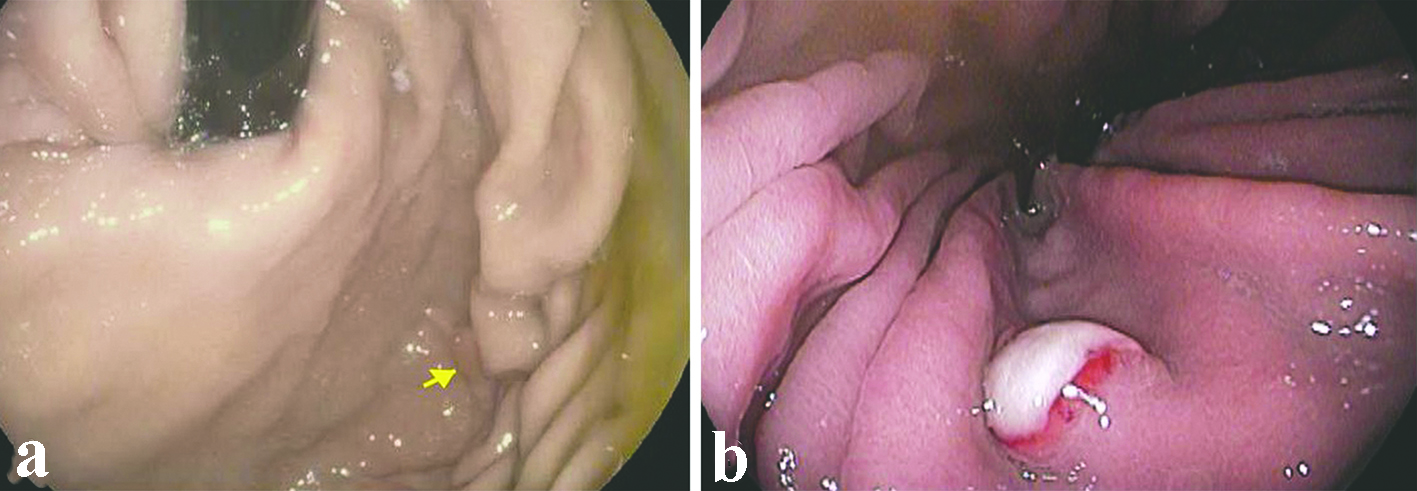 Click for large image | Figure 1. Representative endoscopic appearance of metastatic renal cell carcinoma as gastric mucosal lesions. (a) Metastatic renal cell carcinoma presented as a 0.4 cm polyp (arrow) without ulceration in the gastric body (case 2). (b) Metastatic renal cell carcinoma presented as a 0.8 cm sessile polyp with ulcerated surface in the gastric body (case 4). |
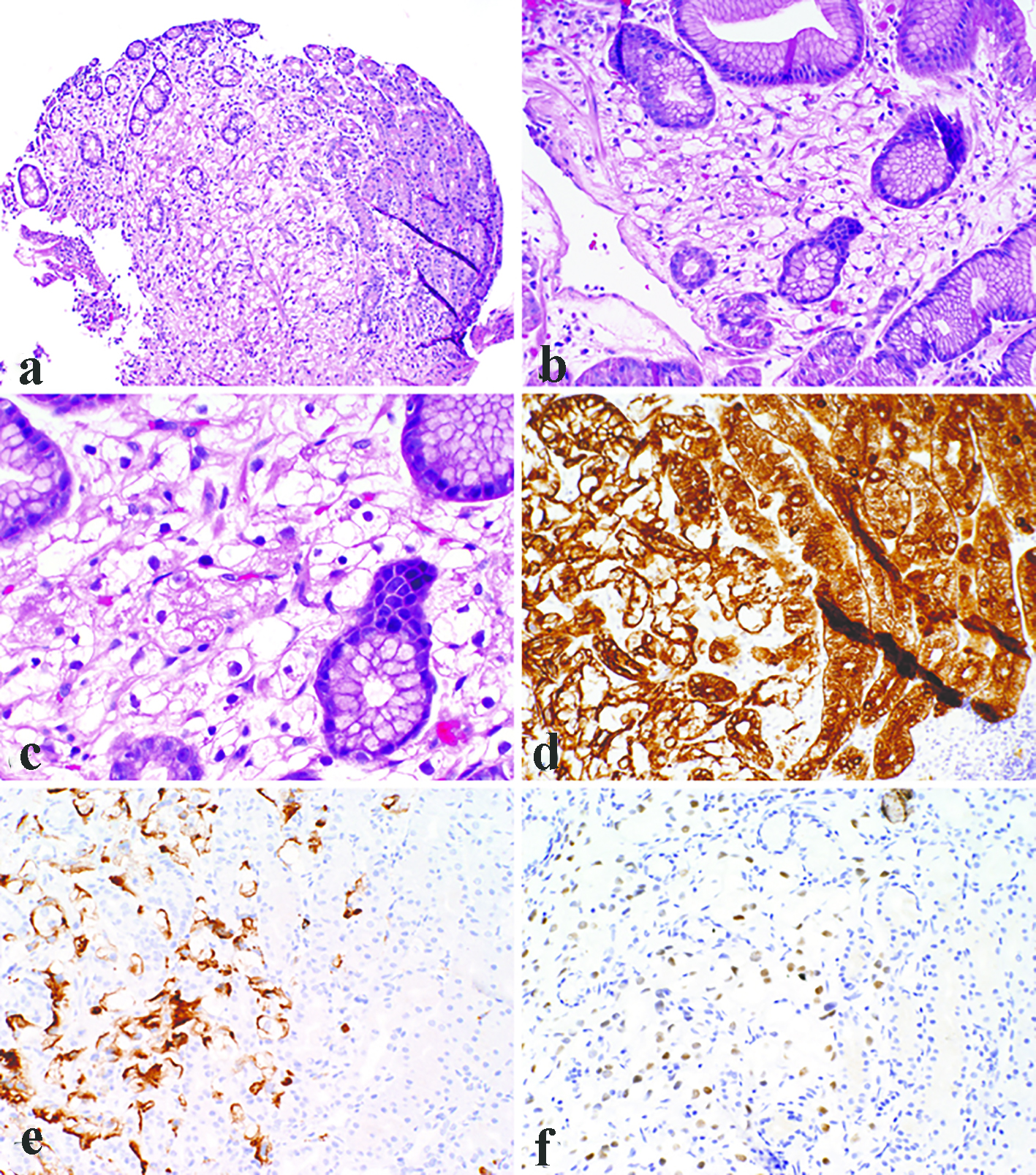 Click for large image | Figure 2. Representative photomicrographs of metastatic clear cell renal cell carcinoma as gastric mucosal lesions. The lesions demonstrated a bland clear cell proliferation within the lamina propria (a). At the interface between the carcinoma and benign gastric mucosa, the clear cells infiltrated among gastric glands without gland destruction (b). The individual tumor cells showed cytoplasmic vacuoles, small nuclei, nuclear membrane irregularity, and occasional small pinpoint nucleoli (c). Immunohistochemistry demonstrated immunoreactivity for pancytokeratin (d), RCC (e), and PAX8 (f). (a, × 100; b, d, e, f, × 200; c, × 400). |
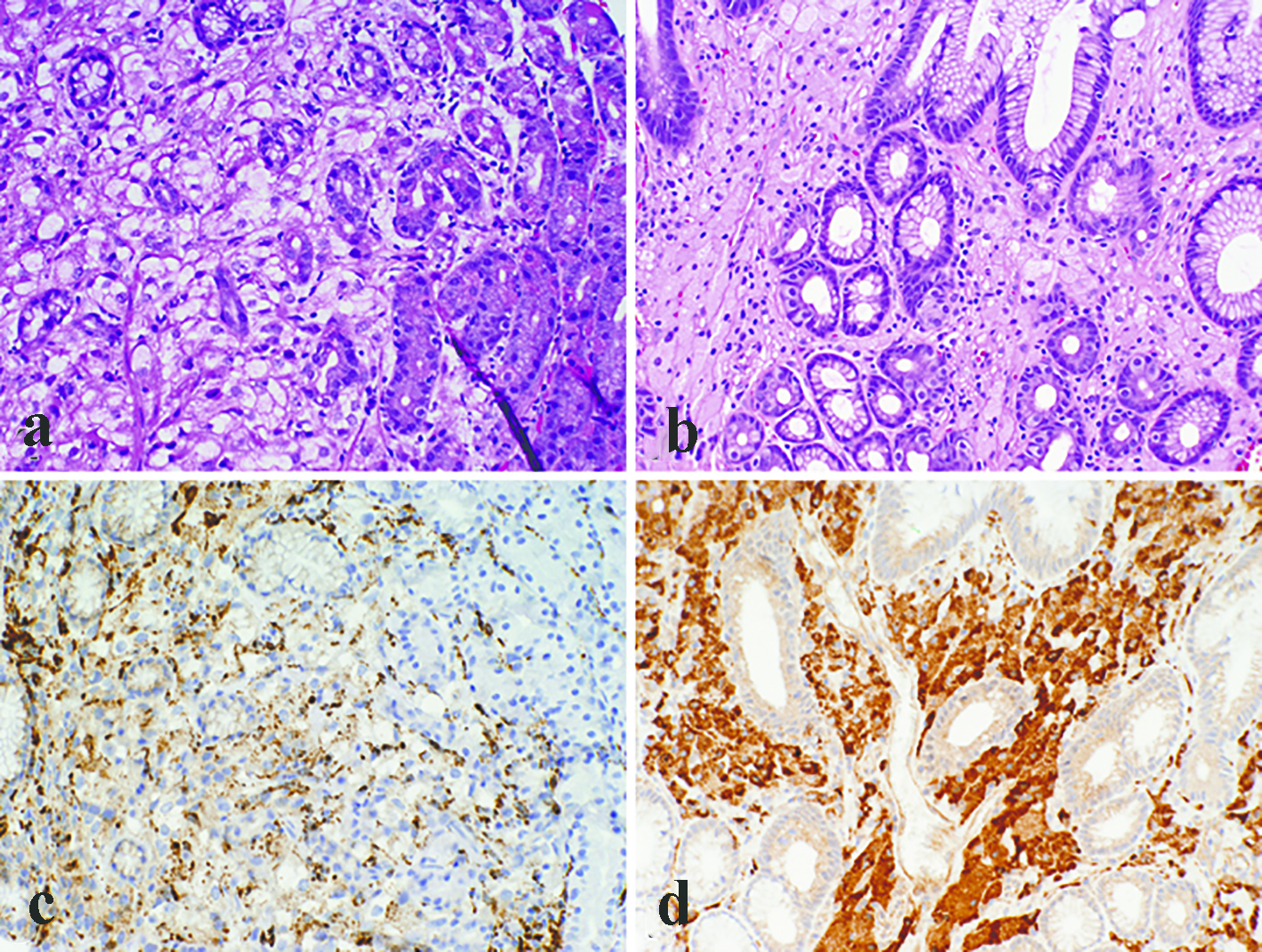 Click for large image | Figure 3. Metastatic renal cell carcinoma with many foamy histiocytes intermingled with the tumor cells (a). However, at least focal nuclear atypia was appreciated in the tumor cells, and immunohistochemistry for CD68 showed patchy staining (c). In contrast, gastric xanthomas were completely devoid of atypia and the cytoplasm was foamy rather than clear (b). Immunoreactivity for CD68 was strong and diffuse in xanthomas (d) (a, b, c, d, × 200). |
| Discussion | ▴Top |
RCC is diagnosed at an advanced stage in up to a quarter of patients. Even in patients with localized disease, a third will develop metastasis after resection [10]. Despite such a high incidence of metastasis, there is a low propensity for RCC to metastasize to the stomach [1, 5, 7, 11-15]. Most metastatic RCCs in the stomach present as large ulcerated mass lesions. There are rare case reports of metastatic RCC presenting as solitary subcentimeter gastric lesion [6, 8, 9]. Therefore, metastatic RCC is unlikely in the differential diagnosis for small solitary gastric lesions. Furthermore, a long interval between primary RCC diagnosis and gastric metastasis is very common. For most metastatic carcinomas (e.g. lung and breast) involving the stomach, the mean interval between the diagnoses of the primary tumor and the gastric metastasis is 1.3 years (range 0 - 4.7 years), with half of the patients having gastric metastasis within a year [4]. In contrast, gastric metastasis attributable to RCC occurs after a significantly longer interval, with a mean interval of 6.5 years (range 0.1 - 20 years) [5]. As a result, in patients with a remote history of RCC, such a history may be overlooked at the time of endoscopy. In our patients, the interval between RCC diagnosis and gastric metastasis ranged from 5 to 10 years. The history of RCC was provided in only two of the three patients. In addition, it is even rarer to have the gastric metastasis be the diagnostic presentation of a carcinoma. This has been reported in primary tumors from breast and lung [2]. In our case series, there were two patients who did not carry a history of RCC, and the diagnosis was established based on the endoscopic gastric biopsies. Therefore, it is of importance to increase the awareness among pathologists of this rare presentation of metastatic RCC as small solitary gastric mucosal lesions, so that this entity will be at least included in the differential diagnosis given the appropriate histopathologic appearance, even if the history of RCC is not provided.
The endoscopic differential diagnosis for small gastric lesions is quite broad [6]. Given the wide differential of subcentimeter gastric lesions, it is difficult to distinguish these on endoscopy. In general, gastric lesions/polyps can be separated into a few basic categories: epithelial lesions (hyperplastic/inflammatory polyp, hamartomatous polyp, fundic gland polyp, adenoma, neuroendocrine tumor), mesenchymal proliferation (gastrointestinal stromal tumor, leiomyoma, inflammatory fibroid polyp), infiltrative lesions (xanthoma, lymphoid proliferation), and malignancies. Malignancies of any primary can also be found in the stomach with a range of endoscopic appearances. The classic “bull’s-eye sign” (lesion with central depression) can be seen, but in less than half of the cases [2]. Subcentimeter lesions are especially less likely to display these classic findings such as “bull’s-eye sign” or even ulceration. In the four previously reported cases, one lesion showed erosion, and three lesions had benign sessile or polypoid appearance [6-9]. In our series, two patients presented with classic findings of a sessile lesion with ulceration and friability (cases 3 and 4); however, in two patients, the gastric metastasis showed more bland endoscopic appearances of 4 - 6 mm sessile polyps (cases 1 and 2). This stresses the importance to sample all subcentimeter lesions despite the endoscopic appearance. The American Society of Gastrointestinal Endoscopy suggests all solitary gastric polyps should be sampled (whether by biopsy or polypectomy). Only polyps suspicious for fundic gland polyp > 1 cm and hyperplastic polyp > 0.5 cm should undergo polypectomy. The guideline for multiple polyps is removing the largest and sampling the smaller polyps. These guidelines are suggestions rather than recommendations due to lack of evidence on how to handle gastric polyps. However, the society stresses the importance of communication with the pathologists [16]. The importance of adequate communication is highlighted by Carmack et al in their description of correlating endoscopic findings with histology [17].
Histologically, though metastatic RCC can be easily differentiated from other common subcentimeter gastric lesions such as hyperplastic polyps and fundic gland polyps, the bland nature of tumor cells and involvement of lamina propria may histologically resemble xanthomatous lesions of the stomach. Gastric xanthomas consist of an accumulation of periodic acid-Schiff (PAS)-negative macrophages with vacuolated sudanophilic lipid-rich cytoplasm. The polygonal macrophages exhibit a distinct cell membrane and a small centrally located round or oval nucleus. They are completely devoid of atypia and lie in a pavement-like arrangement, usually in the upper third of the mucosa. The clear cell RCC cells are also lipid-rich and may exhibit similar morphological features, but the cytoplasm is usually clear rather than foamy as in xanthoma cells. At the interface to normal mucosa, the metastatic RCC cells usually infiltrate among gastric glands without destruction, further mimicking the growth pattern of gastric xanthomas. However, enlarged hyperchromatic atypical cells can be identified at least focally, and in the center of the metastatic focus, the tumor cells showed destructive, rather than expansile, growth. Such histologic features should promote further immunohistochemical workup to rule out malignancy. In three cases in this series, areas with ulceration and granulation tissue were sampled, which might lead to a diagnosis of hyperplastic/inflammatory polyp if the bland RCC infiltration is inconspicuous in the biopsy. Actually one case in our series was a consult case which carried an initial diagnosis of hyperplastic polyp. The confusion with benign gastric polyps can be further complicated by a remote or unknown history of a renal tumor. In these cases, an immunohistochemistry panel of cytokeratin, RCC markers (CD10, RCC, PAX-8) along with CD68 (for xanthomatous lesions) might be necessary. At least patchy immunoreactivity for cytokeratin was present in all the cases, which will likely lead to a correct diagnosis. However, it is worth pointing out that in two cases, there were abundant macrophages intermingled with metastatic tumor cells. Therefore, immunohistochemistry for histiocytic markers (such as CD68) alone may give rise to a misdiagnosis of xanthoma in such a scenario.
In summary, we present five cases of metastatic RCC to the stomach as a solitary subcentimeter mucosal lesion. The remote or unknown history of RCC, the non-specific endoscopic appearance and the bland histological features may lead to potential diagnostic pitfalls. Our case series outlines the importance of careful endoscopic and histologic examination. It is of importance for both endoscopists and pathologists to be aware of such an unusual presentation of metastatic RCC in the stomach and include metastatic RCC in the differential diagnosis for gastric mucosal polyps with clear cell morphology, so that the potential diagnostic pitfalls will be avoided.
Financial Disclosures
None.
| References | ▴Top |
- Green LK. Hematogenous metastases to the stomach. A review of 67 cases. Cancer. 1990;65(7):1596-1600.
doi - Oda, Kondo H, Yamao T, Saito D, Ono H, Gotoda T, Yamaguchi H, et al. Metastatic tumors to the stomach: analysis of 54 patients diagnosed at endoscopy and 347 autopsy cases. Endoscopy. 2001;33(6):507-510.
doi pubmed - Namikawa T, Hanazaki K. Clinicopathological features and treatment outcomes of metastatic tumors in the stomach. Surg Today. 2014;44(8):1392-1399.
doi pubmed - Campoli PM, Ejima FH, Cardoso DM, Silva OQ, Santana Filho JB, Queiroz Barreto PA, Machado MM, et al. Metastatic cancer to the stomach. Gastric Cancer. 2006;9(1):19-25.
doi pubmed - Pollheimer MJ, Hinterleitner TA, Pollheimer VS, Schlemmer A, Langner C. Renal cell carcinoma metastatic to the stomach: single-centre experience and literature review. BJU Int. 2008;102(3):315-319.
doi pubmed - Thoufeeq MH, Maleki N, Jagirdar N, Rembacken B, Jennings J. Renal cell cancer diagnosed at endoscopy. Case Rep Gastrointest Med. 2012;2012:360560.
- Xu J, Latif S, Wei S. Metastatic renal cell carcinoma presenting as gastric polyps: A case report and review of the literature. Int J Surg Case Rep. 2012;3(12):601-604.
doi pubmed - Kim MY, Jung HY, Choi KD, Song HJ, Lee JH, Kim DH, Choi KS, et al. Solitary synchronous metastatic gastric cancer arising from t1b renal cell carcinoma: a case report and systematic review. Gut Liver. 2012;6(3):388-394.
doi pubmed - Saidi RF, Remine SG. Isolated gastric metastasis from renal cell carcinoma 10 years after radical nephrectomy. J Gastroenterol Hepatol. 2007;22(1):143-144.
doi pubmed - Cohen HT, McGovern FJ. Renal-cell carcinoma. N Engl J Med. 2005;353(23):2477-2490.
doi pubmed - Kumcu E, Gonultas M, Unverdi H, Hucumenoglu S. Gastric metastasis of a renal cell carcinoma presenting as a polypoid mass. Endoscopy. 2014;46(Suppl 1 UCTN):E464.
- Akay E, Kala M, Karaman H. Gastric metastasis of renal cell carcinoma 20 years after radical nephrectomy. Turk J Urol. 2016;42(2):104-107.
doi pubmed - Sakurai K, Muguruma K, Yamazoe S, Kimura K, Toyokawa T, Amano R, Kubo N, et al. Gastric metastasis from renal cell c-arcinoma with gastrointestinal bleeding: a case report and review of the literature. Int Surg. 2014;99(1):86-90.
doi pubmed - Yamamoto D, Hamada Y, Okazaki S, Kawakami K, Kanzaki S, Yamamoto C, Yamamoto M. Metastatic gastric tumor from renal cell carcinoma. Gastric Cancer. 2009;12(3):170-173.
doi pubmed - Sugasawa H, Ichikura T, Ono S, Tsujimoto H, Hiraki S, Sakamoto N, Yaguchi Y, et al. Isolated gastric metastasis from renal cell carcinoma 19 years after radical nephrectomy. Int J Clin Oncol. 2010;15(2):196-200.
doi pubmed - Sharaf RN, Shergill AK, Odze RD, Krinsky ML, Fukami N, Jain R, Appalaneni V, et al. Endoscopic mucosal tissue sampling. Gastrointest Endosc. 2013;78(2):216-224.
doi pubmed - Carmack SW, Genta RM, Graham DY, Lauwers GY. Management of gastric polyps: a pathology-based guide for gastroenterologists. Nat Rev Gastroenterol Hepatol. 2009;6(6):331-341.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


