| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Original Article
Volume 16, Number 3, June 2023, pages 157-164
Independent Predictors and Causes of Thirty-Day Gastrointestinal Readmissions Following COVID-19-Related Hospitalizations: Analysis of the National Readmission Database
Robert Kwei-Nsoroa, h, Bashar Attarb, Hafeez Shakac, Pius Ojemolona, Muhammad Sanaa, Abdul Tawab Shakad, Naveen Baskarane, Philip Kanemof, Mohankumar Doraiswamyg
aDepartment of Internal Medicine, John H Stroger Jr Hospital of Cook County, Chicago, IL, USA
bDivision of Gastroenterology and Hepatology, John H Stroger Jr Hospital of Cook County, Chicago, IL, USA
cDivision of Hospital Medicine, John H Stroger Jr Hospital of Cook County, Chicago, IL, USA
dDepartment of Medicine, Windsor University School of Medicine, St Kitts, West Indies
eDivision of Hospital Medicine, University of Florida, Gainesville, FL, USA
fDivision of Hospital Medicine, Rapides Regional Medical Center, Alexandria, LA, USA
gDivision of Nephrology Critical Care, Mercy Hospital, Fort Smith, AR, USA
hCorresponding Author: Robert Kwei-Nsoro, Department of Internal Medicine, John H. Stroger, Jr. Hospital of Cook County, Chicago, IL 60612, USA
Manuscript submitted March 30, 2023, accepted May 2, 2023, published online June 11, 2023
Short title: GI-Related 30-Day Readmission Following COVID-19
doi: https://doi.org/10.14740/gr1623
| Abstract | ▴Top |
Background: The coronavirus disease 2019 (COVID-19) pandemic led to significant mortality and morbidity in the United States. The burden of COVID-19 was not limited to the respiratory tract alone but had significant extrapulmonary manifestations. We decided to examine the causes, predictors, and outcomes of gastrointestinal (GI)-related causes of 30-day readmission following index COVID-19 hospitalization.
Methods: We used the National Readmission Database (NRD) from 2020 to identify hospitalizations among adults with principal diagnosis of COVID-19. We identified GI-related hospitalizations within 30 days of index admission after excluding elective and traumatic admissions. We identified the top causes of GI-related readmission, and the outcomes of these hospitalizations. We used a multivariate Cox regression analysis to identify the independent predictors of readmission.
Results: Among 1,024,492 index hospitalizations with a primary diagnosis of COVID-19 in the 2020 NRD database, 644,903 were included in the 30-day readmission study. Of these 3,276 (0.5%) were readmitted in 30 days due to primary GI causes. The top five causes of readmissions we identified in this study were GI bleeding, intestinal obstruction, acute diverticulitis, acute pancreatitis, and acute cholecystitis. Multivariate Cox regression analysis done adjusting for confounders showed that renal failure, alcohol abuse, and peptic ulcer disease were associated with increased odds of 30-day readmission from GI-related causes.
Conclusions: GI manifestations of COVID-19 are not uncommon and remain an important cause of readmission. Targeted interventions addressing the modifiable predictors of readmission identified will be beneficial in reducing the burden on already limited healthcare resources.
Keywords: COVID-19; 30-day readmission; GI bleeding; Intestinal obstruction; Acute pancreatitis
| Introduction | ▴Top |
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes coronavirus disease 2019 (COVID-19) and led to a global pandemic of significant proportions. The clinical course ranges from asymptomatic to life-threatening acute respiratory syndrome with multi-organ involvement [1, 2]. Similar to other coronaviruses, SARS-CoV-2 infects the gastrointestinal (GI) tract [3]. In addition to common GI symptoms such as diarrhea and vomiting, several case reports have described the occurrence of GI bleeding and other inflammatory processes involving the GI tract [4-7]. The recovery course ranges from 2 - 6 weeks based on the severity of the disease; however, little is known about readmissions following COVID-19 hospitalizations particularly GI-related readmissions. The probability of 30-day readmission is a complex interplay of comorbidities, disease severity, and outpatient management. Understanding the causes and independent predictors of 30-day readmission in patients admitted with COVID-19 would allow the healthcare system to focus already limited resources on the modifiable factors to improve patient outcomes. This study aimed to investigate the causes, outcomes, and independent predictors of GI-related 30-day readmission following index COVID-19 hospitalization.
| Materials and Methods | ▴Top |
This was an observational retrospective study involving COVID-19-related adult hospitalizations, derived from the Nationwide Readmissions Database (NRD) for 2020. The NRD provides data for readmission analysis at the national level and is one of the largest readmission databases in the United States [8]. It contains clinical and non-clinical data about patients’ index hospitalization and subsequent readmissions. The NRD contains three main tables which provide information on the disease, patient, and hospital [9]. It includes demographics, admission diagnosis, and discharge information.
The study involved hospitalizations with COVID-19 infection as the principal diagnosis using the International Classification of Diseases, 10th Revision (ICD-10) diagnostic codes (U.071, U.00, U.49, U.50, U.85, J.1282, and B.342). This was deemed as the index hospitalization. We excluded elective hospitalizations as well as hospitalizations with patients less than 18 years. Hospitalizations in December were excluded during the 30-day readmission analysis. We identified one subsequent admission with 30 days and tagged this as readmission. We excluded elective and traumatic admissions during the readmission analysis.
We assessed patient demographics as well as hospital-specific variables from the variables included in the NRD. We also assessed the comorbidity index using the Elixhauser Comorbidity Index (ECI) which has been used in prior Healthcare Cost and Utilization Project (HCUP) database research [10].
Rate and reasons for GI causes of readmission classified as “gastrointestinal diseases” by ICD codes were the primary outcomes of the study. We then assessed independent predictors of readmissions, readmission mortality, total hospital charges (THC) and mean length of stay (LOS).
We analyzed the data using Stata® Version 17 software. We group age into three categories: 18 - 44 years for young adults, 45 - 64 years for middle-aged adults, and 65 years above for the elderly. We compared readmission mortality, THC, and LOS using univariable regression analysis. In order to identify variables associated with readmission to obtain GI predictors of 30-day all-cause readmission, we used a univariable pre-screening model. We screened age categories, sex, hospital location, hospital bed size, mean household income, and the 31 ECI comorbidities. Variables with P value less than 0.1 were included in the final multivariable regression analysis. We set a P value of < 0.05 as the threshold for statistical significance to identify the independent predictors of readmission during the multivariable Cox regression analysis. This has been adopted in prior HCUP database research [11, 12].
Ethical compliance
In keeping with other HCUP database research we did not require the Institutional Review Board approval for this study. This was a national database study that had no patient identifiers, and we had no direct contact with human/animal subjects.
| Results | ▴Top |
Among 1,024,492 index hospitalizations with a primary diagnosis of COVID-19 in the 2020 NRD database, 644,903 (62.9%) were included in the 30-day readmission study. Of these, 3,276 (0.5%) were readmitted in 30 days due to primary GI causes. Amongst hospitalizations within 30 days, 56% were male and 44% were female. The mean age of male hospitalizations (66.4 ± 15.1) was significantly lower compared to that of female (69.1 ± 16.1) hospitalizations (P < 0.001). Most of the readmitted cohort were elderly and had more than three comorbidities. Table 1 provides the demographic and clinical characteristics of hospitalizations assessed for 30-day readmission stratified by biological sex.
 Click to view | Table 1. Baseline Characteristics of Patients Evaluated for Gastrointestinal Etiologies of 30-Day Readmissions Following Index COVID-19 Admission Stratified by Biological Sex |
The top five causes of readmissions we identified in this study were GI bleeding, intestinal obstruction, acute diverticulitis, acute pancreatitis, and acute cholecystitis (Table 2). Mortality was higher in the index admission (11.6 %) compared to the 30-day readmission (5.1%). LOS and THC were also higher during index admission compared to 30-day readmission for GI-related hospitalizations (Table 3). There was no difference in outcomes (mortality, LOS and THC) between the male and female sex in the 30-day readmission study (Table 4).
 Click to view | Table 2. The Five Most Common Gastrointestinal Etiologies of 30-Day Readmissions Following Index COVID-19 Admission. |
 Click to view | Table 3. Mortality, Length of Stay, and Total Hospitalization Charges on Index Admission Compared With 30-Day Readmission. |
 Click to view | Table 4. Mortality, Length of Stay, and Total Hospitalization Charges on 30-Day Readmission Stratified by Biological Sex |
Cox regression analysis adjusting for confounders revealed that private insurance (adjusted hazard ratio (aHR): 0.59; 95% confidence interval (CI): 0.49 - 0.72; P <0.001) and obesity (aHR: 0.86; 95% CI: 0.76 - 0.98; P <0.025) were associated with lower odds of 30-day readmission. Congestive heart failure (aHR: 1.39; P < 0.001), renal failure (aHR 3.06; P < 0.001), alcohol abuse (aHR: 2.67; P < 0.001), coagulopathy (aHR: 1.47; P < 0.001), and peptic ulcer disease (aHR: 1.92; P = 0.021), were associated with increased odds of 30-day readmission from GI-related causes shown in the Forrest plot (Fig. 1). Table 5 shows the aHR, CI, and P values of the independent predictors associated with 30-day GI-related readmission following index COVID-19 admission.
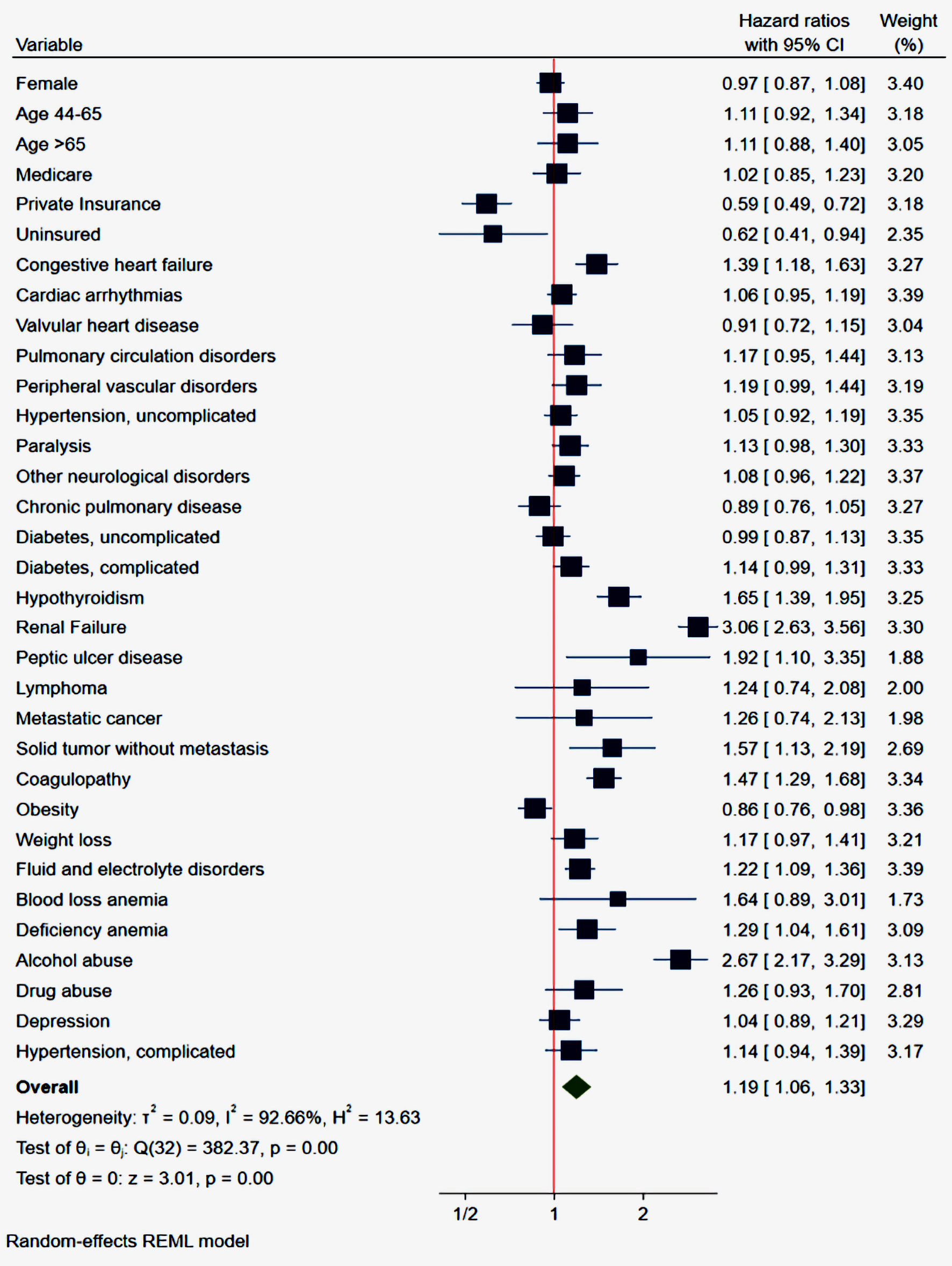 Click for large image | Figure 1. Forrest plot of multivariate analysis of independent predictors associated with 30-day readmissions. CI: confidence interval. |
 Click to view | Table 5. Independent Predictors of 30-Day GI-Related Readmission Following Index COVID-19 Hospitalization |
| Discussion | ▴Top |
This analysis, to the best of our knowledge, is the first observational report from a national representative database on GI-related causes and predictors of readmissions following COVID-19 hospitalizations. The pathophysiology of GI-related injury in COVID-19 is not clearly understood but it is thought to include a combination of direct virus-mediated tissue damage, intestinal edema, diffuse endothelial and submucosal vascular inflammation, and virally mediated alterations to the intestinal microbiome [13-15]. Given the above, there are potentially several plausible processes by which COVID-19 may result in GI sequelae of infection. GI bleeding was the most common cause of 30-day readmission in our study, followed by intestinal obstruction, acute diverticulitis, acute pancreatitis, and acute cholecystitis.
The rate of GI bleeding in patients with COVID-19 was pegged at 2% in a meta-analysis by Marasco et al. Not many studies exist comparing rates of GI bleeding in non-COVID-19 and COVID-19 patients making accurate comparisons difficult. Nonetheless, pooled data on GI bleeding rates in COVID-19 suggest an increased risk when compared to the general population [16]. The mechanisms thought to cause GI bleeding in COVID-19 include a combination of use of systemic corticosteroids, use of antiplatelets and anticoagulants given evidence of thrombotic risks in COVID-19 patients, inflammation induced coagulopathy, and direct mucosal damage from SARS-CoV-2 as it can infect enteric cells via angiotensin-converting enzyme 2 (ACE), a viral binding site abundantly expressed in the enterocytes [15, 17].
Intestinal obstruction following COVID-19 has been reported in a few case reports [18-20]. One was of a patient who presented with large bowel obstruction due to colonic strictures, subsequent colectomy showed chronic active inflammation on histology [18]. Bowel ischemia and COVID-19 colitis have been reported in other case reports which can be potential causes of bowel obstruction [21-23]. The exact mechanism of the intestinal obstruction following COVID-19 is unclear; it may, however, be related to post-inflammatory changes from the direct viral invasion of enterocytes or electrolyte derangement with subsequent adynamic ileus from COVID-19-induced diarrhea and vomiting.
Karime et al studied 81 patients with first-time acute diverticulitis following COVID-19 at a large academic center [5]. This and a few other case reports have suggested an association between diverticulitis and COVID-19 [24, 25]. The pathophysiology of acute diverticulitis remains unclear, but some studies have reported an association between alteration of the intestinal microbiome and chronic inflammation as part of the processes involved in the development of acute diverticulitis [26, 27]. Given the generalized inflammatory state noted in COVID-19 and the direct viral invasion of enterocytes, it is possible to speculate that COVID-19 can lead to acute diverticulitis in susceptible individuals via inflammation-mediated tissue damage [5].
Viruses including varicella-zoster, human immunodeficiency virus (HIV), mumps, and cytomegaloviruses have been implicated as causes of acute pancreatitis. SARS-CoV-2 has been isolated from pancreatic specimens during autopsies of infected patients [28]. It is entirely plausible that other causes (alcohol, gallstones) might have been implicated here, but the association between COVID-19 and acute pancreatitis is difficult to ignore as many case reports have suggested an association between COVID-19 and acute pancreatitis [6, 29, 30].
Acalculous cholecystitis usually results from stasis, gallbladder ischemia, and endothelial injury. It has been described in association with COVID-19 in some case reports [7, 31, 32]. SARS-CoV-2 has been identified in gallbladder and bile specimens following cholecystectomy in patients with COVID-19 [31, 33]. This gives credence to the theory that direct viral invasion of the gallbladder wall may lead to acute acalculous cholecystitis via virus-mediated tissue damage and diffuse endothelial and submucosal vascular inflammation [13, 15].
Several comorbidities are associated with a higher risk of GI-related readmission after recent COVID-19 hospitalization as shown in Figure 1. The strongest predictor of GI-related 30-day readmission following index COIVD admission is renal failure (hazard ratio (HR): 3.06; 95% CI: 2.63 - 3.56). Renal failure has a well-established association with GI hemorrhage [34], which is the most common GI etiology of readmission after COVID-19 hospitalization in our analysis (Table 2). Several mechanisms have been postulated in literature for the increased rates of GI bleeding in the renal failure cohort. Uremic toxins lead to direct platelet dysfunction leading to an increased risk of bleeding despite normal levels of platelets [35]. Similarly, uremia has been associated with dysfunctional von Willebrand factor which raises the risk of bleeding as well [36]. Renal failure has also been established as an independent risk factor for cardiovascular disease [37], and frequent use of antiplatelet agents and anticoagulants for cardiovascular disease may increase the bleeding risk in such population. Lastly, vascular malformation (angiodysplasia) frequently seen in renal failure patients increases the risk of GI bleeding four to six times as compared to healthy adults [38]. In summary, the virus-mediated damage to the intestinal mucosa in addition to renal failure-associated dysfunction can explain the higher risk of GI hemorrhage in short-term follow-up after COVID-19.
Alcohol use is the second strongest predictor with a greater than two-fold increase in the likelihood of GI-related 30-day readmission (HR: 2.67; 95% CI: 2.17 - 3.29) after recent COVID-19 infection. The most common mechanisms reported are alcohol-induced gastritis, acute pancreatitis, chronic liver disease complicated by esophageal varices, and hemostatic disease associated with dysfunction of coagulation cascades [39]. Peptic ulcer disease regardless of etiology was also associated with an increased risk of readmission (HR: 1.92; 95% CI: 1.10 - 3.35).
Other significant but weak predictors of increased 30-day readmissions included congestive heart failure (HR: 1.39; 95% CI: 1.18 - 1.63), hypothyroidism (HR: 1.65; 95% CI: 1.39 - 1.95), solid tumor without metastasis (HR: 1.57; 95% CI: 1.13 - 2.19), coagulopathy (HR: 1.47; 95% CI: 1.29 - 1.68) and fluid and electrolyte disorders (HR: 1.22; 95% CI: 1.09 - 1.36).
Obesity has been shown to be associated with increased severity and higher mortality among COVID-19 patients [40]. This may be due to the over-expression of ACE receptors associated with obesity [41]. Interestingly however, we found obesity to be associated with lower odds of 30-day readmission in our study. There is currently no data that support reduced odds of 30-day readmission for GI-related conditions amongst patients with obesity except a weak association with lower odds of 30-day readmission amongst obese patients admitted with portal venous thrombosis [11]. The reason for this observation is unclear to us.
Private insurance has been shown to be associated with reduced odds of 30-day readmissions for some prevalent cardiopulmonary conditions and GI bleeding [42, 43]. Hospitalizations with private insurance were associated with reduced odds of 30-day readmission in our study. This is likely due to restrictions in care and unequal access to newer technologies based on insurance coverage.
There were some limitations in our study despite this being a comprehensive study of a nationally representative sample. This was an observational study using the NRD database and thus our findings cannot establish causation. Also, the database limits the ability to capture patient-level data regarding the underlying etiology of the various causes of readmissions identified as some may not necessarily be related to COVID-19. Despite the limitations, the data were from a large national database and allowed us to provide insights into our primary and secondary outcomes to statistically significant levels.
Extra-pulmonary manifestations of COVID-19 are not uncommon and remain an important cause of readmission. We have with this analysis shown the most common causes of GI-related readmissions and thrown more light on the proposed pathophysiological mechanisms underlying COVID-19 and GI tract pathology. Targeted interventions addressing the independent predictors of readmissions identified here may help reduce overall healthcare costs.
Acknowledgments
The completion of this research work would not have been possible without the support of my wife Akua Ofori-Karikari.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
This was a National Readmission Database study hence informed consent is not applicable to this study.
Author Contributions
Robert Kwei-Nsoro participated in the writing and review of this manuscript. Bashar Attar, Pius Ojemolon, Abdul Tawab Shaka, Naveen Baskaran, Philip Kanemo, and Mohankumar Doraiswamy participated in the review of this manuscript. Hafeez Shaka and Muhammad Sana participated in the writing this manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069.
doi pubmed pmc - Puelles VG, Lutgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, Chilla S, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020;383(6):590-592.
doi pubmed pmc - Openshaw PJ. Crossing barriers: infections of the lung and the gut. Mucosal Immunol. 2009;2(2):100-102.
doi pubmed pmc - Sanku K, Siddiqui AH, Paul V, Ali M. An unusual case of gastrointestinal bleeding in a patient with COVID-19. Cureus. 2021;13(3):e13901.
doi pubmed pmc - Karime C, Travers P, Ouni A, Francis D. Association Between Coronavirus Disease 2019 and Acute Complicated Diverticulitis. Cureus. 2023;15(1):e33252.
doi pubmed pmc - Alves AM, Yvamoto EY, Marzinotto MAN, Teixeira ACS, Carrilho FJ. SARS-CoV-2 leading to acute pancreatitis: an unusual presentation. Braz J Infect Dis. 2020;24(6):561-564.
doi pubmed pmc - Alhassan SM, Iqbal P, Fikrey L, Mohamed Ibrahim MI, Qamar MS, Chaponda M, Munir W. Post COVID 19 acute acalculous cholecystitis raising the possibility of underlying dysregulated immune response, a case report. Ann Med Surg (Lond). 2020;60:434-437.
doi pubmed pmc - HCUP Nationwide Readmission Database (NRD). Healthcare Cost and Utilization Project (HCUP). Agency for Healthcare Research and Quality, Rockville, MD. 2018. https://www.hcup-us.ahrq.gov/nrdoverview.jsp.
- Barrett M, Steiner C, Andrews R, Kassed C, Nagamine M. Methodological issues when studying readmissions and revisits using hospital administrative data. 2011. HCUP Methods Series Report # 2011-01. U.S. Agency for Healthcare Research and Quality. Online March 9, 2011. Available: http://www.hcupus.ahrq.gov/reports/methods/methods.jsp.
- Kwei-Nsoro R, Ojemolon P, Laswi H, Ebhohon E, Ufeh AO, Nieto A, Mir WA, et al. Effect of the COVID-19 pandemic on the epidemiological trends and outcomes of gastrointestinal bleeding: a nationwide study. Proc (Bayl Univ Med Cent). 2023;36(2):145-150.
doi pubmed pmc - Kwei-Nsoro R, Ojemolon P, Laswi H, Ebhohon E, Shaka A, Mir WA, Siddiqui AH, et al. Rates, reasons, and independent predictors of readmissions in portal venous thrombosis hospitalizations in the USA. Gastroenterology Res. 2022;15(5):253-262.
doi pubmed pmc - Shaka H, El-Amir Z, Jamil A, Kwei-Nsoro R, Wani F, Dahiya DS, Kichloo A, et al. Plasmapheresis in hypertriglyceridemia-induced acute pancreatitis. Proc (Bayl Univ Med Cent). 2022;35(6):768-772.
doi pubmed pmc - Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417-1418.
doi pubmed pmc - Mak JWY, Chan FKL, Ng SC. Probiotics and COVID-19: one size does not fit all. Lancet Gastroenterol Hepatol. 2020;5(7):644-645.
doi pubmed pmc - Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, Bikdeli B, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26(7):1017-1032.
doi pubmed - Marasco G, Maida M, Morreale GC, Licata M, Renzulli M, Cremon C, Stanghellini V, et al. Gastrointestinal bleeding in COVID-19 patients: a systematic review with meta-analysis. Can J Gastroenterol Hepatol. 2021;2021:2534975.
doi pubmed pmc - Qi F, Qian S, Zhang S, Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun. 2020;526(1):135-140.
doi pubmed pmc - Yousaf K, Toffaha A, Yasin ALF, Al Naimi N, Ahmed A, Abu Nada M, Yousif M, et al. A rare case of COVID-19 infection leading to colonic stricture: case report and review of literature. Cureus. 2022;14(7):e27043.
doi pubmed pmc - Laski D, Biernat K, Kaska L. Pneumatosis intestinalis due to COVID-19 infection in kidney transplant recipient: a case report. Transplant Proc. 2021;53(4):1215-1218.
doi pubmed pmc - Varshney R, Bansal N, Khanduri A, Gupta J, Gupta R. Colonic gangrene: a sequela of coronavirus disease 2019. Cureus. 2021;13(4):e14687.
doi pubmed pmc - Gartland RM, Velmahos GC. Bowel necrosis in the setting of COVID-19. J Gastrointest Surg. 2020;24(12):2888-2889.
doi pubmed pmc - Shaikh DH, Patel H, Makker J, Badipatla K, Chilimuri S. Colonic ileus, distension, and ischemia due to COVID-19-related colitis: a case report and literature review. Cureus. 2021;13(2):e13236.
doi pubmed pmc - Stawinski P, Dziadkowiec KN, Marcus A. COVID-19-induced colitis: a novel relationship during troubling times. Cureus. 2021;13(6):e15870.
doi pubmed pmc - Weissman S, Belyayeva A, Sharma S, Aziz M, Elias S, Tabibian JH. SARS-CoV-2 and acute diverticulitis: the expanding gastrointestinal manifestations of COVID-19 infection. J Transl Int Med. 2021;9(1):59-60.
doi pubmed pmc - Patel P, Phan E, Pona A, Mao YX. S1625 acute perforated diverticulitis as a potential complication of SARS-CoV-2 (COVID-19). Am J Gastroenterol. 2020;115:S834.
doi - Humes DJ, Spiller RC. Review article: The pathogenesis and management of acute colonic diverticulitis. Aliment Pharmacol Ther. 2014;39(4):359-370.
doi pubmed - Gueimonde M, Ouwehand A, Huhtinen H, Salminen E, Salminen S. Qualitative and quantitative analyses of the bifidobacterial microbiota in the colonic mucosa of patients with colorectal cancer, diverticulitis and inflammatory bowel disease. World J Gastroenterol. 2007;13(29):3985-3989.
doi pubmed pmc - Ding Y, He L, Zhang Q, Huang Z, Che X, Hou J, Wang H, et al. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol. 2004;203(2):622-630.
doi pubmed pmc - Hassani AH, Beheshti A, Almasi F, Ketabi Moghaddam P, Azizi M, Shahrokh S. Unusual gastrointestinal manifestations of COVID-19: two case reports. Gastroenterol Hepatol Bed Bench. 2020;13(4):410-414.
pubmed pmc - Sandhu H, Mallik D, Lokavarapu MJ, Huda F, Basu S. Acute recurrent pancreatitis and COVID-19 infection: a case report with literature review. Cureus. 2021;13(2):e13490.
doi pubmed pmc - Scutari R, Piermatteo L, Ciancio Manuelli M, Iannetta M, Salpini R, Bertoli A, Alteri C, et al. Long-Term SARS-CoV-2 Infection Associated with Viral Dissemination in Different Body Fluids Including Bile in Two Patients with Acute Cholecystitis. Life (Basel). 2020;10(11):302.
doi pubmed pmc - Abaleka FI, Nigussie B, Bedanie G, Mohammed A, Galiboglu S. Acute acalculous cholecystitis due to COVID-19, an unusual presentation. Cureus. 2021;13(6):e15431.
doi pubmed pmc - Balaphas A, Gkoufa K, Meyer J, Peloso A, Bornand A, McKee TA, Toso C, et al. COVID-19 can mimic acute cholecystitis and is associated with the presence of viral RNA in the gallbladder wall. J Hepatol. 2020;73(6):1566-1568.
doi pubmed pmc - Ishigami J, Grams ME, Naik RP, Coresh J, Matsushita K. Chronic kidney disease and risk for gastrointestinal bleeding in the community: the atherosclerosis risk in communities (ARIC) study. Clin J Am Soc Nephrol. 2016;11(10):1735-1743.
doi pubmed pmc - Kaw D, Malhotra D. Platelet dysfunction and end-stage renal disease. Semin Dial. 2006;19(4):317-322.
doi pubmed - Gralnick HR, McKeown LP, Williams SB, Shafer BC, Pierce L. Plasma and platelet von Willebrand factor defects in uremia. Am J Med. 1988;85(6):806-810.
doi pubmed - Vallianou NG, Mitesh S, Gkogkou A, Geladari E. Chronic kidney disease and cardiovascular disease: is there any relationship? Curr Cardiol Rev. 2019;15(1):55-63.
doi pubmed pmc - Zajjari Y, Tamzaourte M, Montasser D, Hassani K, Aatif T, El Kabbaj D, Benyahia M. Gastrointestinal bleeding due to angiodysplasia in patients on hemodialysis: A single-center study. Saudi J Kidney Dis Transpl. 2016;27(4):748-751.
doi pubmed - Flores B, Trivedi HD, Robson SC, Bonder A. Hemostasis, bleeding and thrombosis in liver disease. J Transl Sci. 2017;3(3).
doi pubmed pmc - Singh R, Rathore SS, Khan H, Karale S, Chawla Y, Iqbal K, Bhurwal A, et al. Association of obesity with COVID-19 severity and mortality: an updated systemic review, meta-analysis, and meta-regression. Front Endocrinol (Lausanne). 2022;13:780872.
doi pubmed pmc - Kruglikov IL, Scherer PE. The role of adipocytes and adipocyte-like cells in the severity of COVID-19 infections. Obesity (Silver Spring). 2020;28(7):1187-1190.
doi pubmed pmc - Garg S, Campbell P, Wadhwa V, Anugwom C, Shergill S, Gupta N, et al. Incidence and predictors of 30-day readmission of patients in patients hospitalized with gastrointestinal bleeding: an analysis of national readmission database: 2511. American Journal of Gastroenterology. 2016;111:S1277.
- Ferro EG, Secemsky EA, Wadhera RK, Choi E, Strom JB, Wasfy JH, Wang Y, et al. Patient readmission rates for all insurance types after implementation of the hospital readmissions reduction program. Health Aff (Millwood). 2019;38(4):585-593.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


