| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Review
Volume 13, Number 5, October 2020, pages 163-183
A Focused Review on Advances in Risk Stratification of Malignant Polyps
Enoch Kuoa, b, Kai Wanga, b, Xiuli Liua, c
aDepartment of Pathology, Immunology & Laboratory Medicine, College of Medicine, University of Florida, Gainesville, FL 32610, USA
bBoth authors contributed equally to this manuscript.
cCorresponding Author: Xiuli Liu, Department of Pathology, Immunology & Laboratory Medicine, College of Medicine, University of Florida, Gainesville, FL 32610, USA
Manuscript submitted September 15, 2020, accepted October 20, 2020, published online October 31, 2020
Short title: Risk Stratification of Malignant Polyps
doi: https://doi.org/10.14740/gr1329
- Abstract
- Introduction
- Polyp Definition and Types
- Two Major Colorectal Cancer Pathways
- Polyp Classification by the Paris System
- Endoscopic Removal of Polyps: Polypectomy (in Whole or Piecemeal), Endoscopic Mucosal Resection (EMR) and Endoscopic Submucosal Dissection (ESD)
- Macroscopic Examination and Sampling of Polypectomy, EMR and ESD Specimens
- Malignant Polyp Definition and Diagnostic Criteria
- Prognostic Factors for Malignant Polyps That Trigger Oncological Resection
- Other Special Issues
- Conclusion
- References
| Abstract | ▴Top |
Colorectal cancer is the third most common cancer in both men and women in the United States, with most cases arising from precursor adenomatous polyps. Colorectal malignant polyps are defined as cancerous polyps that consist of tumor cells invading through the muscularis mucosae into the underlying submucosa (pT1 tumor). It has been reported that approximately 0.5-8.3% of colorectal polyps are malignant polyps, and the potential for lymph node metastasis in these polyps ranges from 8.5% to 16.1%. Due to their clinical significance, recognition of malignant polyps is critical for clinical teams to make treatment decisions and establish appropriate surveillance schedules after local excision of the polyps. There is a rapidly developing interest in malignant polyps within the literature as a result of an increasing number of identifiable adverse histologic features and recent advancements in endoscopic treatment techniques. The purpose of this paper is to have a focused review of the recent histopathologic literature of malignant polyps.
Keywords: Malignant polyp; Diagnostic criteria; Prognostic factors; Risk stratification
| Introduction | ▴Top |
Colorectal cancer is the third most common cancer in both men and women in the United States. Fortunately, the incidence of colorectal cancer has been on the decline due to the widespread use of screening programs to detect precursor lesions and early cancers [1]. Most colorectal cancers arise from adenomatous polyps, with remaining cases arising from serrated polyps or de novo [2]. The adenoma-carcinoma sequence is an indolent but progressive process, which may take many years after a stepwise accumulation of genetic alterations. Approximately 0.5-8.3% of colorectal polyps are reported to be malignant polyps [3], and the potential for lymph node metastasis from malignant polyps ranges from 8.5% to 16.1% [4, 5]. Therefore, careful histopathologic examination of malignant polyps is critical to optimize treatment and surveillance plans for patients following the initial local excision. There is growing literature regarding the different histopathologic features of malignant colonic polyps and their management. The purpose of this paper is to have a focused review on the recent histopathology literature of malignant polyps.
| Polyp Definition and Types | ▴Top |
Colorectal polyps are masses of colonic tissue that extend from the inner mucosa lining of the colon into the lumen, and they may be categorized as neoplastic or non-neoplastic (hamartomatous and inflammatory polyps). Neoplastic polyps have two major histologic types: conventional adenomas and serrated polyps. Serrated polyps are further categorized into hyperplastic polyps, sessile serrated lesions and traditional serrated adenomas. Endoscopically, polyps can also be morphologically described as either pedunculated or sessile polyps. Pedunculated polyps are polyps that attach to the colonic mucosa by a stalk of variable length. In contrast, sessile polyps grow in a flattened arrangement over the mucosa with little separation of lesional epithelium from the background normal mucosal surface [6]. The distinction between pedunculated and sessile malignant polyps is relevant because sessile malignant polyps have increased risks for lymph node metastasis, positive endoscopic resection margins and worse outcomes compared to pedunculated malignant polyps [7-10]. The question of “how to risk stratify endoscopically resected sessile malignant polyps” becomes a more prominent issue in clinical practice as more morphologic and histologic features are identified in malignant polyps and are confirmed to be associated with nodal metastases, distant metastases and/or survival.
| Two Major Colorectal Cancer Pathways | ▴Top |
Two major types of genomic instability have been described in colorectal carcinogenesis: chromosomal instability and microsatellite instability [11, 12]. The majority of sporadic colorectal cancers (approximately 85%) exhibit chromosomal instability, in which defective chromosomal segregation in mitosis and cell cycle dysregulation leads to gains and/or losses of chromosomal segments, chromosomal rearrangements and loss of heterozygosity [13-17]. The chromosomal instability pathway is characterized by the accumulation of mutations in specific oncogenes, including KRAS proto-oncogene GTPase (KRAS), B-Raf proto-oncogene serine/threonine kinase (BRAF) and tumor suppressor genes such as adenomatous polyposis (APC) gene and tumor protein p53 (TP53) [14, 18, 19]. Traditionally, the adenoma-carcinoma sequence first begins with inactivating APC gene mutations, which translate to low-grade conventional adenomatous dysplasia. Activating KRAS mutations occur later in the adenomatous stage and are associated with polyp size progression and grade of dysplasia. Subsequent deletion of the deleted in colorectal cancer (DCC) gene on chromosome 18q and inactivation of TP53 on chromosome 17p results in malignancy [20, 21].
The remaining colorectal cancer cases are caused by defective or loss of DNA mismatch repair (MMR) proteins, which lead to an increased mutation rate and malignancies characterized by high-frequency microsatellite instability (MSI) [22]. Microsatellites are DNA sequences composed of repetitive 1-6 base pair tandem repeats that have an increased risk for slippage by the DNA polymerase during replication [23]. When MMR proteins are defective or absent, the progressive erroneous insertion and/or deletions of nucleotides within microsatellite sequences are left uncorrected by MMR proteins and lead to longer or shorter alleles from other repair mechanisms [24]. The aberrant DNA sequences can then be detected by polymerase chain reaction (PCR) methods using a panel of 5 - 10 markers. Historically, five microsatellite markers have been used to examine the microsatellite status of a tumor. These markers include two mononucleotide (BAT26 and BAT25) and three dinucleotide (D2S123, D5S346 and D17S250) repeats [25]. A pentaplex panel of five mononucleotide repeats is commercially available, and a hexaplex panel of six mononucleotide repeats has recently been developed [26]. Tumors are classified as microsatellite instability high (MSI-H) if more than 30% of the markers exhibit microsatellite instability [27]. Tumors with detected instability in less than 30% of the markers are classified as microsatellite instability low (MSI-L), and tumors with no instability are defined as microsatellite stable (MSS). Studies have identified four key proteins involved in DNA mismatch repair: mutL homolog 1 (MLH1), PMS1 homolog 2 (PMS2), mutS homolog 2 (MSH2) and mutS homolog 6 (MSH6). MLH1 binds to PMS2 to form a functional heterodimer involved in DNA repair. Similarly, MSH2 and MSH6 bind to form a heterodimer. Loss of expression or function in one of these four proteins will lead to deficiency in DNA mismatch repair and subsequent MSI [28-31].
While the traditional adenoma-carcinoma sequence is associated with conventional adenomatous dysplasia, a distinct serrated pathway to colorectal cancer has also been described. Approximately 15-30% of all colorectal cancers arise from neoplastic serrated polyps. Sessile serrated adenoma/polyp (SSA/P) and traditional serrated adenoma (TSA) are the two major precursor lesions [32, 33]. In the serrated pathway, mutually exclusive activating mutations of either BRAF or KRAS initiate the development of serrated polyps. SSA/Ps are only associated with BRAF mutations, while TSA can have either a KRAS or BRAF mutation. SSA/P progresses to dysplasia through epigenetic silencing of genes, specifically by hypermethylation of CpG islands in the promoter region of tumor suppressor genes. Tumors that progress through this method are described as having a CpG island methylator phenotype (CIMP). CIMP plays a critical role in the progression of SSA/P to invasive colorectal adenocarcinoma through inactivation of MLH1 transcription and aberrant activation of the WNT signaling pathway. MLH1 gene promoter hypermethylation with resultant loss of MLH1 and MSI-H is found in about 75% of SSA/P with dysplasia [34-36]. The remaining 25% of SSA/P that develop into colorectal carcinoma occur through alterations in the WNT signaling pathway and TP53 mutations [37-40]. In comparison, TSAs do not have hypermethylation of the MLH1 promoter but instead show O(6)-methylguanine-DNA methyltransferase (MGMT) hypermethylation [41]. Progression from TSA to colorectal adenocarcinoma occurs by aberrant activation of the WNT pathway and CIMP [37, 42, 43].
| Polyp Classification by the Paris System | ▴Top |
Polyps can be classified by macroscopic or endoscopic appearance using the Paris international classification (Table 1) (Fig. 1) [44]. The inter-observer agreement among gastroenterologists and gastrointestinal surgeons using the Paris system for polyp classification has been examined. In one study, a total of 85 short endoscopic video clips depicting polyps were created and assessed by seven expert endoscopists according to the Paris classification system. The interobserver agreement of the Paris classification among gastroenterologists and gastrointestinal surgeons was moderate with a Fleiss kappa of 0.42 and a mean pairwise agreement of 67%. The proportion of lesions assessed as “flat” by the experts ranged between 13% and 40% (P < 0.001) [45]. In another study, an online video-based survey was sent to gastroenterologists and gastrointestinal surgeons affiliated with six tertiary academic centers. The survey consisted of high-definition video clips (30 - 60 s) of six complex colorectal polyps (one malignant) with associated clinical histories. Accuracy for Paris classification was 47.5% with moderate interobserver agreement (k = 0.48). Surgeons had the least accuracy in assigning Paris classification compared to gastroenterologists (28.7% vs. 66.0%, P < 0.0001) [46]. These studies suggest that the use of the Paris classification system in daily practice is questionable and unsuitable for comparative endoscopic research. However, it is possible that not all of the participating surgeons performed endoscopies or made endoscopic diagnoses in routine practice. It is important to compare the endoscopic diagnoses of pre-resection lesions to the histopathologic findings in endoscopic/surgical resection specimens in order to improve endoscopic diagnostic abilities.
 Click to view | Table 1. The Paris Classification of Colorectal Neoplastic Lesions |
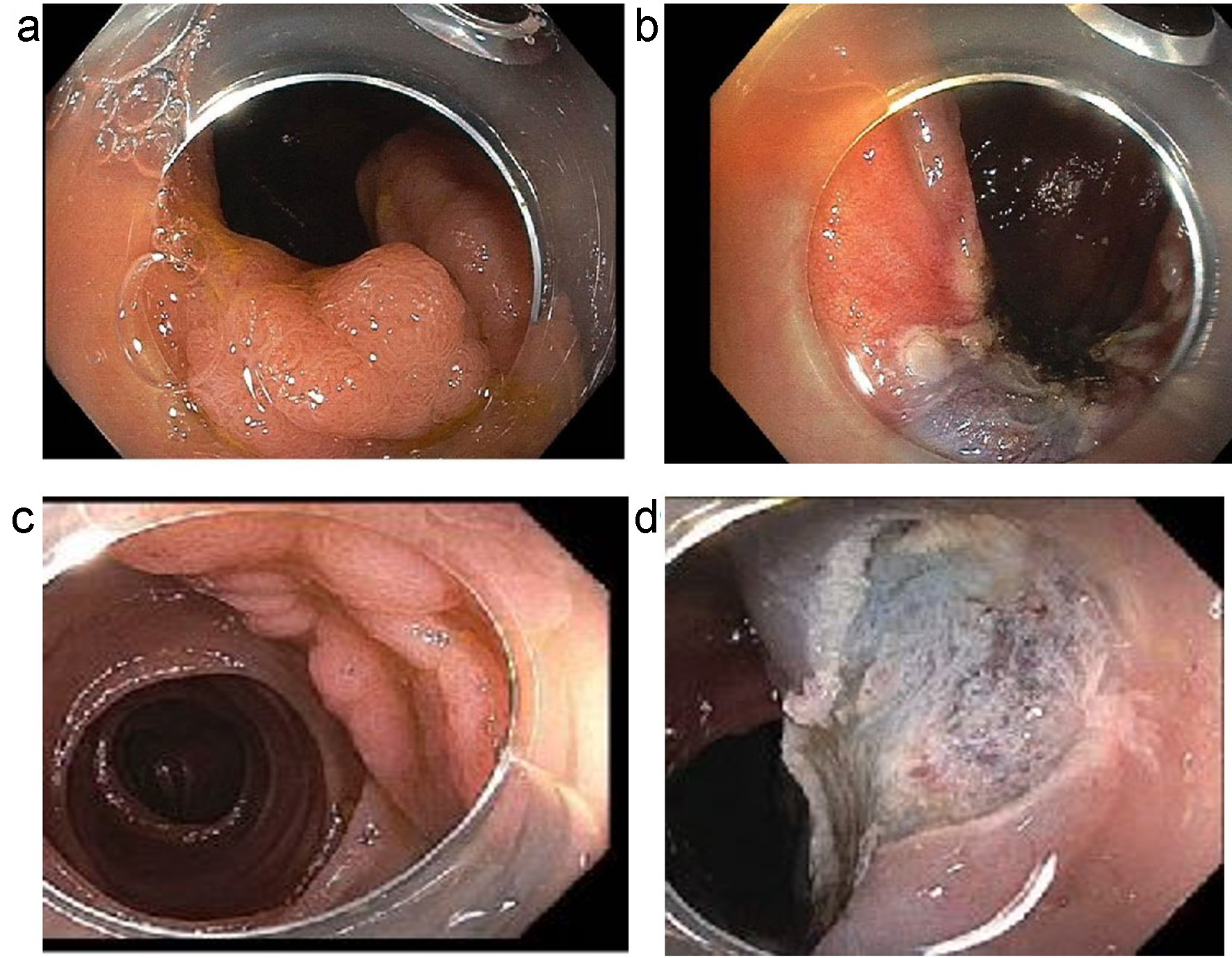 Click for large image | Figure 1. (a, b) An example of a laterally spreading Paris 0-IIa lesion with slight elevation. (a) Before polypectomy. (b) Post-polypectomy. (c, d) An example of a sessile slightly elevated lesion (Paris IIa-Is). (c) Before polypectomy. (d) Post-polypectomy. |
| Endoscopic Removal of Polyps: Polypectomy (in Whole or Piecemeal), Endoscopic Mucosal Resection (EMR) and Endoscopic Submucosal Dissection (ESD) | ▴Top |
Currently, the main endoscopic techniques for the removal of colon polyps are polypectomy, EMR and ESD. Approximately 80-90% of adenomas are less than 1 cm and, therefore, more easily amenable to complete excision with conventional snare polypectomy. The risk of developing advanced neoplasia following polypectomy was estimated at 0.6% [47]. A large retrospective cohort study found that malignant pedunculated colorectal polyps with polyp head invasion can be safely treated by endoscopic polypectomy alone [48]. The treatment of larger sessile polyps can be more challenging and require more advanced techniques, such as EMR or ESD.
EMR was developed for removal of sessile polyps that are confined to the mucosa and the submucosa, as well as dysplastic polyps that are larger than 1 cm but less than 2 cm in size. Several EMR techniques have been described including injection-assisted EMR, ligation-assisted EMR and underwater EMR. Among them, injection-assisted EMR is the most commonly used technique. It involves an initial submucosal injection of saline, or other suitable injectate, to elevate the lesion and facilitate its removal from the deeper layers of the colon by electrocautery snare [49-51].
ESD is performed for lesions with high likelihood of cancer invading the superficial submucosa and for lesions that cannot be removed by EMR due to fibrosis in the submucosa or post-EMR recurrences. This procedure uses saline or other suitable injectate to lift the polyp and then the mucosal incision and submucosal dissection are performed with specialized endoscopic electrosurgical knives [51, 52]. These techniques are associated with a slightly higher risk of serious complications such as bleeding and perforation compared to polypectomy [53].
| Macroscopic Examination and Sampling of Polypectomy, EMR and ESD Specimens | ▴Top |
Proper handling of the polyp specimen is essential for thorough histologic examination of the polyps. The polypectomy specimen needs adequate formalin in the endoscopy room for fixation, and a minimum of 2 - 3 h of fixation is required. It is recommended that polyps larger than 1.5 cm should be fixed overnight [54]. The deep or vertical margin needs to be identified and inked when grossing a polyp. In pedunculated polyps, this margin is the most distal end of the stalk (Fig. 2). For sessile polyps, the deep margin is the line of resection or the base of the polyp. Polyps larger than 0.9 cm should be serially sectioned through a sagittal plane that is perpendicular to the stalk or base of excision (Fig. 2), and the sections should be carefully examined for invasion. Sections of the polyp should be submitted with the cut surfaces facing down so that an entire sagittal section of the polyp in relation to its deep/vertical margin can be visualized on the slides. Polyps less than 0.9 cm in size should be bisected, and both halves should be submitted in a cassette with the cut surfaces facing down. Very small polyps can be entirely submitted in one cassette without sectioning.
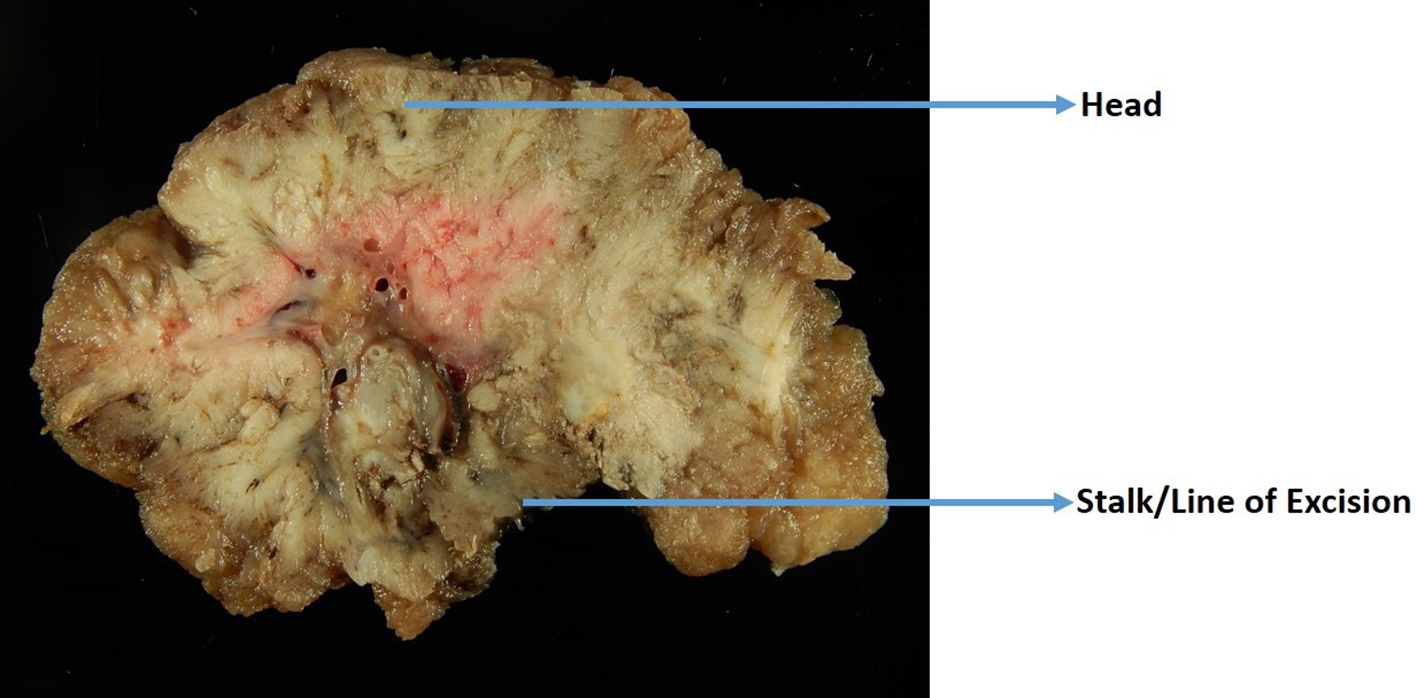 Click for large image | Figure 2. A gross photo of a large polyp that is cut perpendicular to the line of excision. |
ESD specimens are usually received pinned out flat on a cork or foam board by the endoscopist (Fig. 3). All ESD specimens have peripheral and deep/vertical resection margins that should be evaluated. If the specimen has been oriented by the endoscopist or surgeon, a diagram of the specimen can be drawn to document where sections are taken. The size of the whole specimen and the size of the lesion should be measured, as well as the distance to the nearest peripheral margin. If the orientation of the specimen is provided, the deep margin, the designated peripheral mucosal margin and the opposing peripheral mucosal margin should be inked in three different colors, with the deep margin inked black for better visualization. If the specimen is received unoriented, a single ink color can be used for all the peripheral margins. The specimen needs to be sectioned into 2 - 3 mm sections in thickness, and the sections should be perpendicular to the long axis for greater sampling. During serial sectioning, the deepest extent of the lesion should be measured and documented. The distances of the lesion to the nearest peripheral and deep margins should be measured and documented. If the lesion is within 1 cm from a peripheral margin, perpendicular sections of the margin should be taken in order to show the margin in relation to the lesion. If the lesion is greater than 1 cm away from the peripheral margin, a thin en-face section of the peripheral margin is acceptable. The specimen should be entirely submitted if the specimen is not overwhelmingly large. However, if the specimen is too large to be entirely submitted, the closest peripheral margin and the entire blocked out lesion should be submitted at minimum. For very large specimens, one representative section per 1 cm of the lesion should be submitted, including sections with the closest peripheral margin, the closest deep margin and the deepest extent of invasion. If a definitive invasive component is not identified on the initial blocks, the remaining lesion should be entirely submitted for histological examination to confirm the absence of invasion.
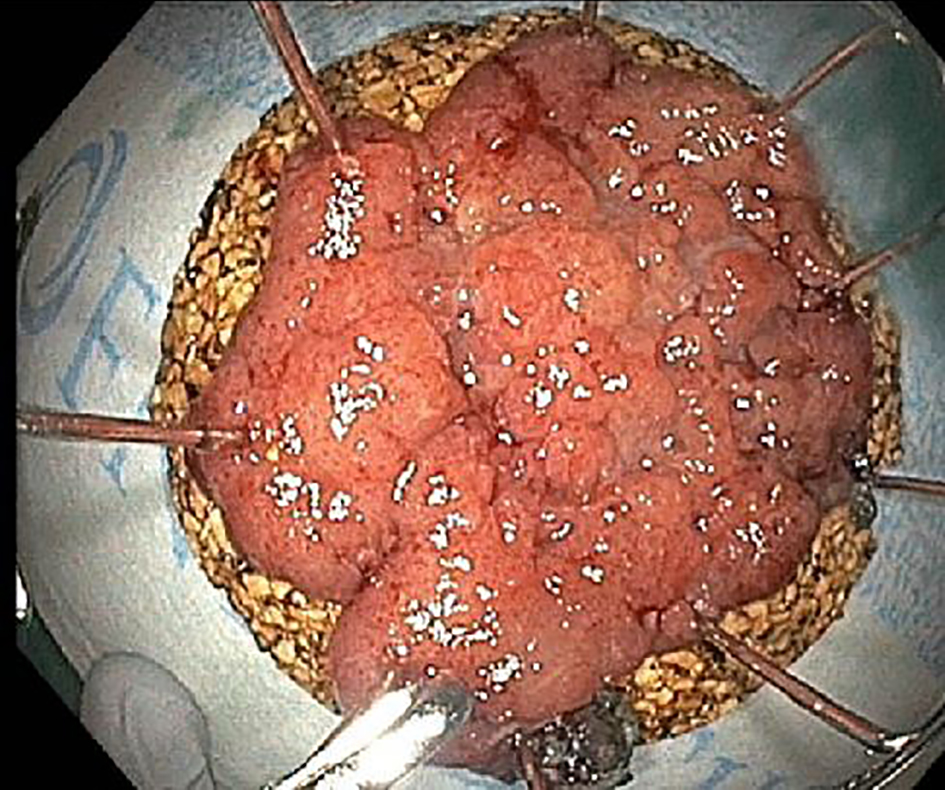 Click for large image | Figure 3. Endoscopic dissection specimens are usually pinned out flat on a cork or foam board by the endoscopist. All ESD specimens have peripheral and deep/vertical resection margins. ESD: endoscopic submucosal dissection. |
| Malignant Polyp Definition and Diagnostic Criteria | ▴Top |
A malignant polyp is defined as a polyp with cancer cells invading into the submucosa through the muscularis mucosae without extension beyond the submucosa [6]. Thus, a malignant polyp may also be referred to as an early colorectal carcinoma (T1) [55]. Diagnosis of a malignant polyp in a well-oriented resection specimen is usually straightforward by identifying malignant glands/cells infiltrating through the muscularis mucosae into, but not beyond, the submucosa. This infiltration process is often associated with desmoplastic change of the submucosal tissue. The glands are often irregular and incomplete, with high-grade cytological atypia and/or complex architecture. Tumor cells are rounded with high nuclear to cytoplasmic (N/C) ratio, significant pleomorphism and hyperchromatic nuclei. Prominent nucleoli may be present, and tumor cells may have marked nuclear stratification. Commonly, there will be increased mitoses with atypical mitotic figures. In addition to high-grade cytological atypia, the lesion may show architectural complexity including back-to-back tubules, cribriforming glands and/or formation of solid nests. Neoplastic glands may also contain intraluminal necrosis. In cases of invasive adenocarcinoma arising in a polyp, well-oriented sections will show glands with high-grade cytologic features and complex architecture extending from the polyp into the submucosa. Desmoplasia is usually seen in areas where the tumor invades through the muscularis mucosae into the submucosa. Most cases of malignant polyps are obvious and simple to diagnose (Fig. 4).
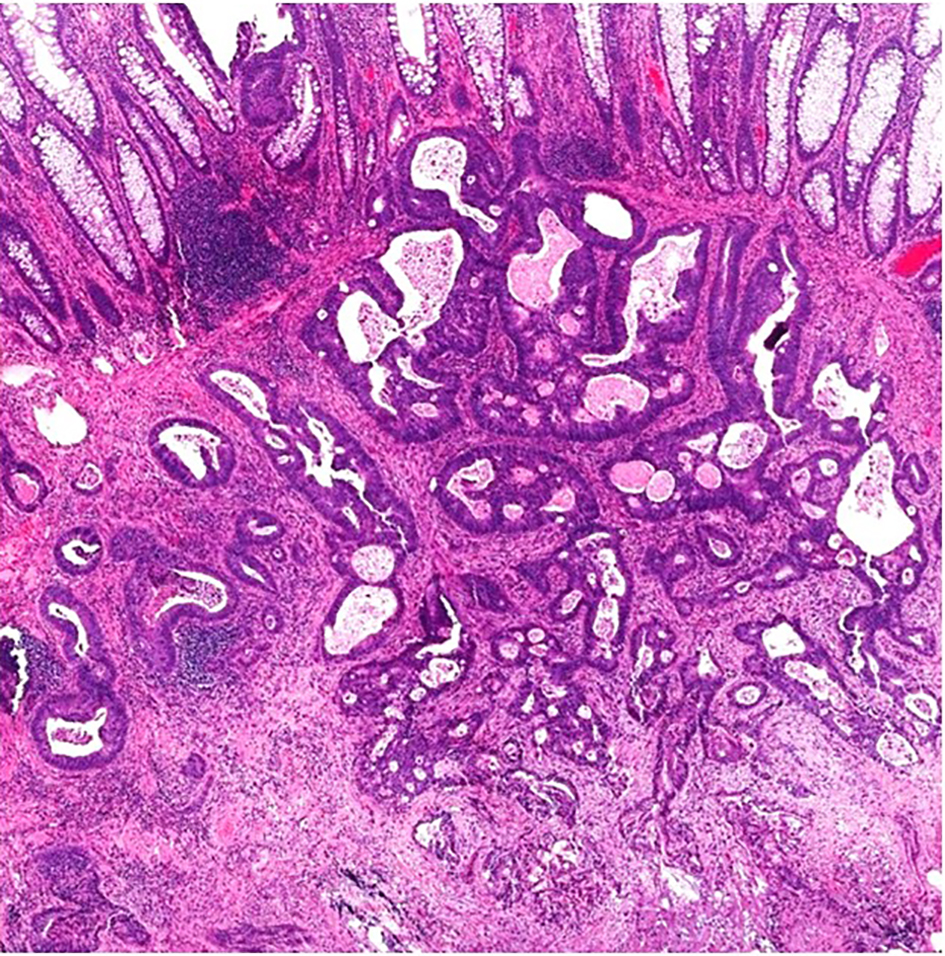 Click for large image | Figure 4. A malignant polyp in a well-oriented specimen showing irregular, ill-formed glands with luminal necrosis below the muscularis mucosae. There is prominent desmoplasia in the deep aspect of the lesion. |
One potential diagnostic pitfall in the diagnosis of a “malignant” polyp is the misplacement of dysplastic epithelium into the submucosa of a pedunculated polyp due to mucosa injury with epithelial herniation into the submucosa. Submucosal epithelial misplacement usually occurs in pedunculated polyps that have round, smooth contours. The misplaced glands are often round and have histomorphology similar to the background adenomatous component of the polyp. A direct connection between the misplaced glands and the surface adenomatous component may be seen (Fig. 5). Submucosal epithelial misplacement usually has lamina propria present around the crypts and associated hemosiderin within the stroma, indicative of prior chronic injury. In contrast, the glands in an invasive adenocarcinoma are irregularly shaped with a haphazard growth pattern, and show high cytologic abnormalities. Additionally, invasive adenocarcinoma may have small abortive glands, single cells and desmoplasia. Submucosal epithelial misplacement may have mucin pools, and these are commonly round, smooth, and lined by dysplastic epithelium at the periphery. In comparison, the presence of floating neoplastic epithelium or single cells within pools of mucin is supportive of an invasive mucinous adenocarcinoma. Table 2 summarizes the features differentiating these two entities. In difficult cases, deeper levels and re-embedding the block may be helpful. Table 3 summarizes the essential elements that should be included in a surgical pathology report for a malignant polyp.
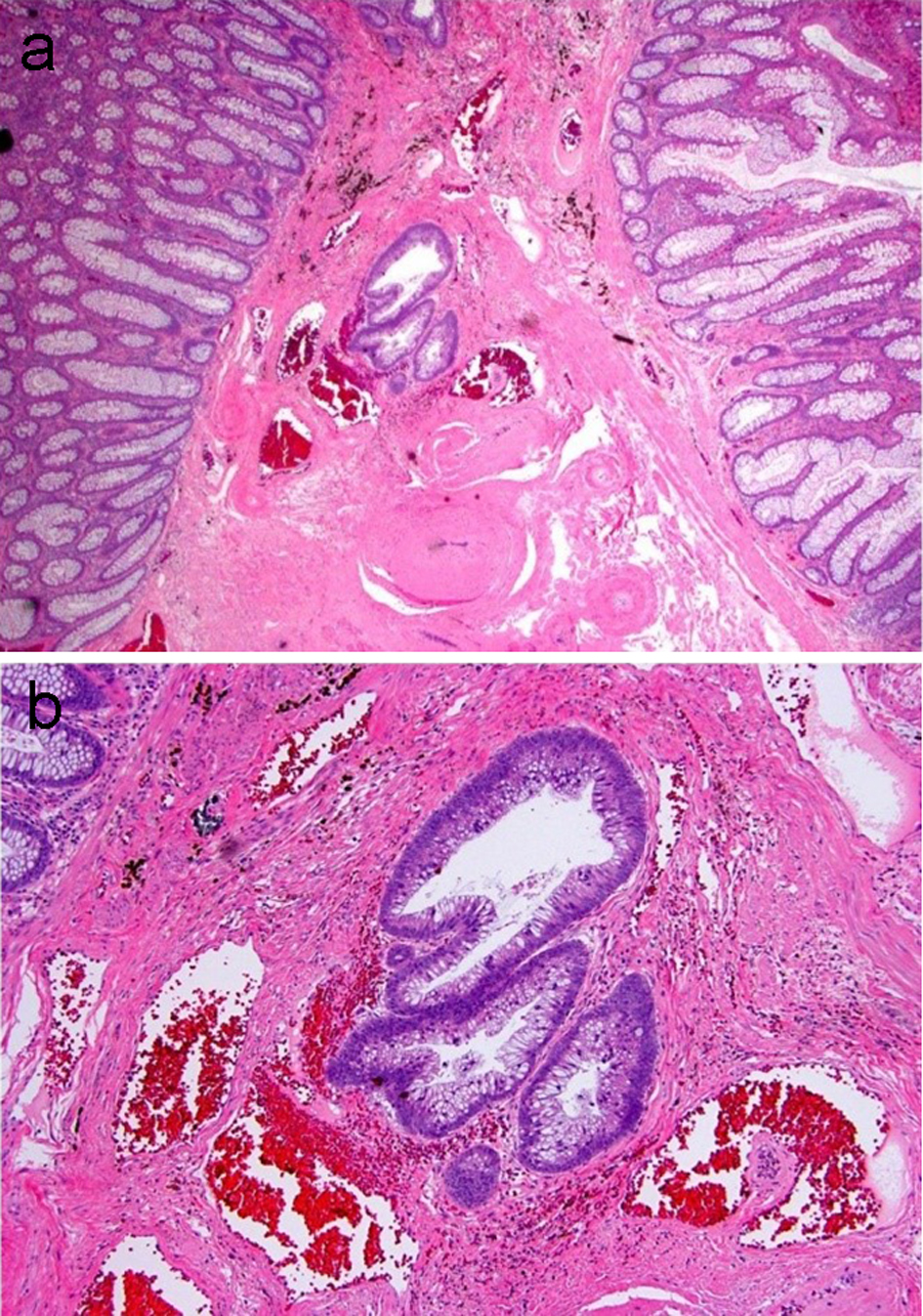 Click for large image | Figure 5. An adenomatous polyp with misplaced adenomatous epithelium within the submucosa. (a) Well-formed adenomatous glands are present below the muscularis mucosa. (b) Higher magnification reveals glands with low-grade dysplasia and a rounded contour surrounded by lamina propria. Hemosiderin is present in the adjacent stroma. |
 Click to view | Table 2. Histologic Features Distinguishing Misplacement of Dysplastic Epithelium and Submucosal Invasion |
 Click to view | Table 3. Essential Elements in the Surgical Pathology Report for a Malignant Polyp |
| Prognostic Factors for Malignant Polyps That Trigger Oncological Resection | ▴Top |
Haggitt level
The depth of invasion in malignant polyps is an important predictive factor for lymph node metastasis. The level of invasion in a pedunculated malignant polyp has been classified by Haggitt et al in 1985 [56]. According to this classification system, pedunculated polyps can be classified into five levels: levels 0 - 4 (Fig. 6). Level 0 indicates that cancer cells are limited to the mucosa but do not penetrate through the muscularis mucosa (carcinoma in situ or intramucosal carcinoma). Level 1 means the adenocarcinoma invades through the muscularis mucosae into the submucosa of the polyp head (Fig. 7). Level 2 denotes invasion of adenocarcinoma into the neck of the polyp (the junction of the head and the stalk of the polyp). Level 3 designates invasion of adenocarcinoma into the stalk of the polyp; however, the tumor is still confined to the polyp stalk. Level 4 is extension of adenocarcinoma below the polyp stalk into the submucosa of the colorectal wall, but not into the muscularis propria. For sessile polyps, any degree of invasion was defined as level 4 by the Haggitt schema. The correlation between Haggitt level, risk of lymph node metastasis and outcome has been reported. A retrospective cohort study found that the incidence of lymph node metastasis was 0.0% (0/101) in patients with polyp head invasion (Haggitt level 1), compared to 6.2% (8/129) in patients with stalk invasion (Haggitt level 3). The authors concluded that malignant pedunculated polyps pathologically diagnosed with polyp head invasion can be managed by endoscopic treatment alone [48]. One study by Kyzer found that only malignant polyps with Haggitt level 4 invasion required resection [57]. Nivatvongs et al reported that the overall incidence of lymph node metastasis for malignant pedunculated polyp was 6%. There was no lymph node metastasis if the depth of invasion was limited to the head (level 1), neck (level 2) and stalk of the polyp (level 3). However, if the depth of invasion reached the base of the stalk (level 4), then the incidence of lymph node metastasis was up to 27%. The author concluded that the most significant risk factor for lymph node metastasis in a malignant polyp was tumor invasion into the submucosa of the bowel wall (level 4) [58]. Therefore, these findings suggest that only malignant polyps with invasion into Haggitt level 4 require a formal resection. The use of Haggitt levels in malignant polyps has been recently succeeded by submucosal invasion measurements pioneered by Kudo et al [59] and Kikuchi et al [60].
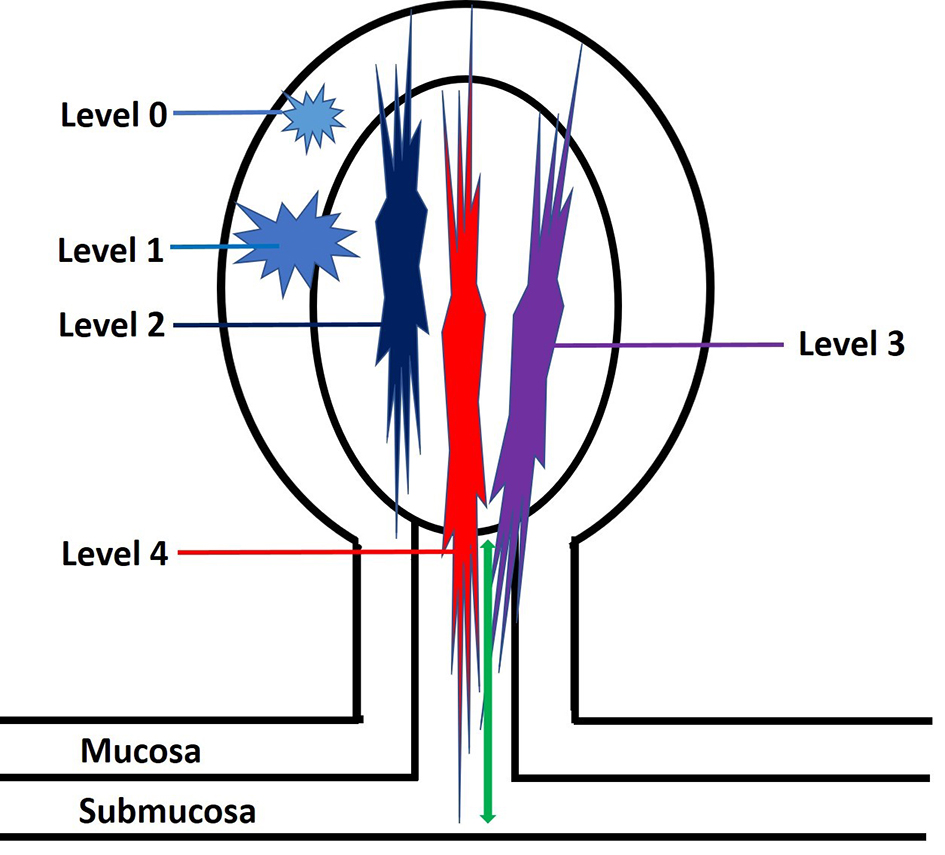 Click for large image | Figure 6. An illustration of Haggitt levels for pedunculated polyps. Level 0: tumor is limited to the mucosa without invasion into the submucosa. Level 1: tumor invades into the submucosa but is limited to the head of the polyp. Level 2: tumor invades into the polyp’s neck (the junction of the head and the stalk). Level 3: tumor invades into the stalk. Level 4: tumor invades beyond the stalk of the polyp but remains above the muscularis propria. The depth of submucosal invasion is measured from the polyp’s neck to the deepest portion of the invasion (green arrow). |
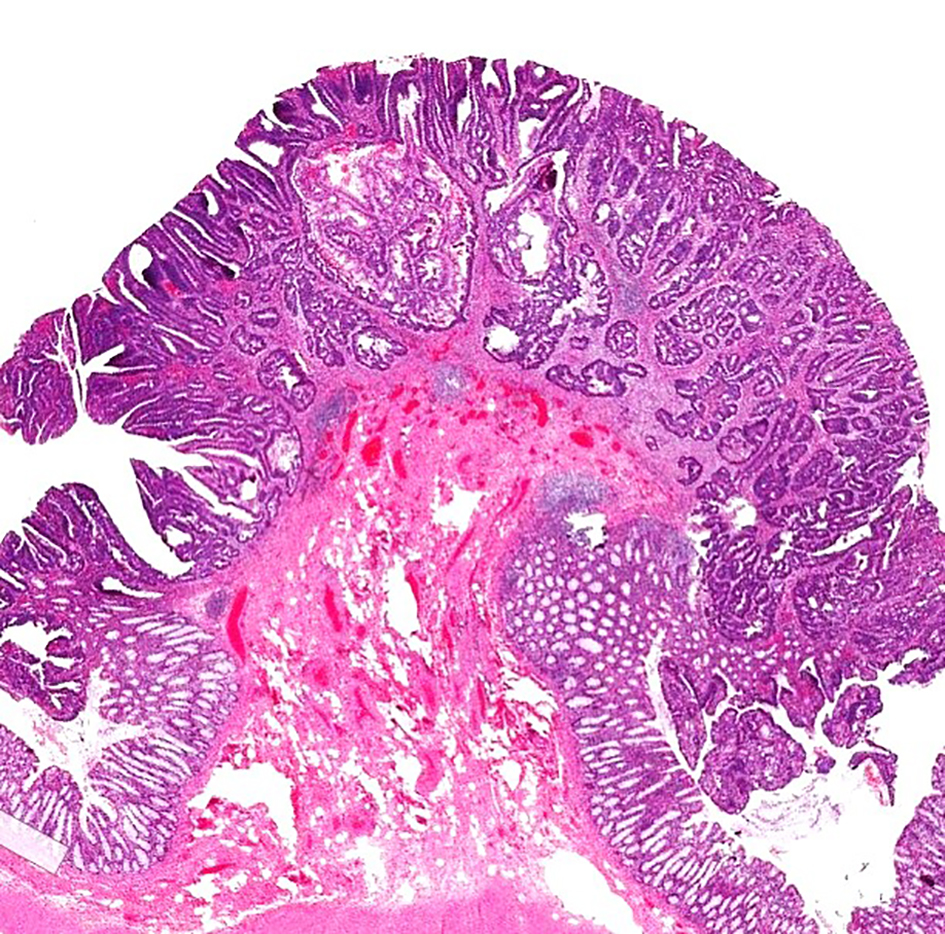 Click for large image | Figure 7. H&E stain of a pedunculated malignant polyp. The tumor invades into the submucosa but is confined to the head of the polyp (Haggitt level 1). H&E: hematoxylin and eosin. |
Submucosal invasion measurement and cutoff
Submucosal invasion of adenocarcinoma in sessile polyps is further classified into three levels by Kudo et al [59] and Kikuchi et al [60]. Sessile polyps with invasion into the upper third of the submucosa are designated as Sm1. Sm2 indicates that a sessile polyp has invasion into the middle third of the submucosa. Sm3 indicates that the invasive component extends into the lower third of the submucosa. One study showed that Sm3 sessile lesions are associated with a significantly higher risk of lymph node metastasis, which can be up to 23% [61]. This classification system has been further modified to be a more practical way of measuring the degree of submucosal invasion from the muscularis mucosae [62] (Figs. 8a and 9a). However, if the muscularis mucosae layer is completely obliterated by tumor, the measurement is taken from the most superficial aspect of invasive adenocarcinoma down to its deepest extent (Figs. 8b and 9b). For pedunculated polyps, Haggitt level 2 (the junction of the polyp’s head and its stalk) is used as the baseline, and this is also referred to as the “Haggitt line”. The depth of submucosal invasion is measured from the Haggitt line to the deepest portion of invasion (Fig. 6). This study found that the incidence of lymph node metastasis was zero for pedunculated malignant polyps with stalk invasion and also for cases with depth of submucosal invasion less than 3,000 µm without lymphatic invasion. For nonpedunculated submucosal invasive colorectal adenocarcinomas, the rate of lymph node metastasis was zero if the depth of submucosal invasion was less than 1,000 µm [62].
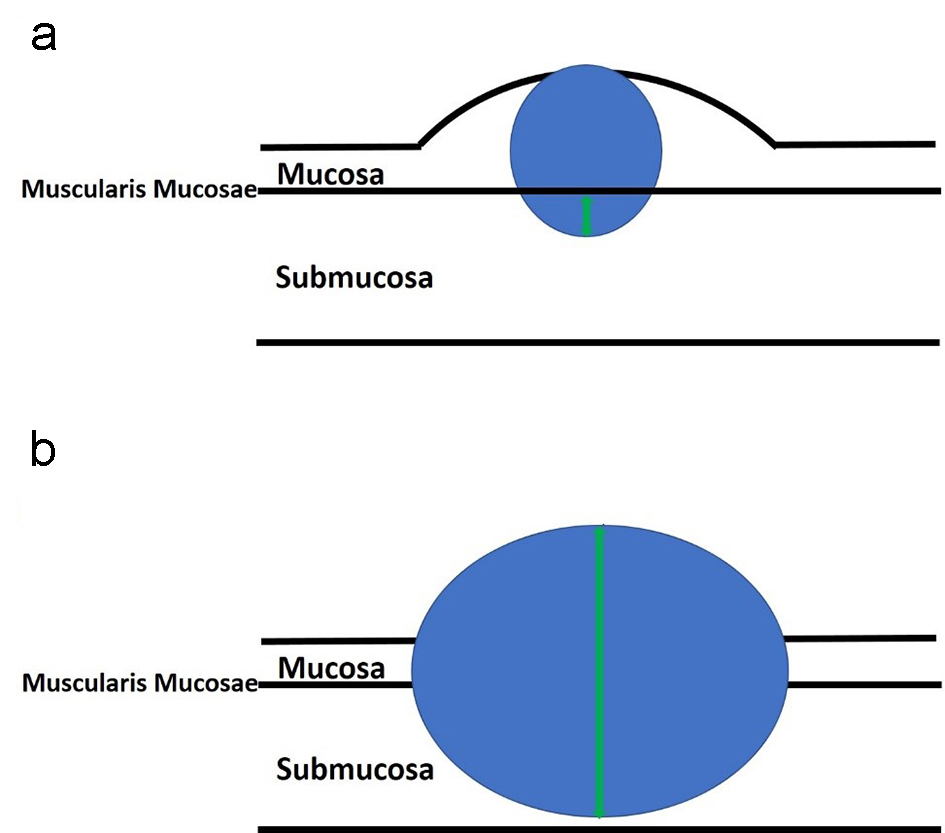 Click for large image | Figure 8. An illustration of sessile polyps and measurements for the depth of submucosal invasion. Sessile polyps grow in a flattened conformation over the mucosa. (a) The depth of submucosal invasion is usually measured from the muscularis mucosae to the deepest aspect of invasion (green arrow). (b) However, if the muscularis mucosae layer is completely obliterated by the tumor, the measurement is taken from the most superficial portion of invasion down to its deepest extent of invasion (green arrow). |
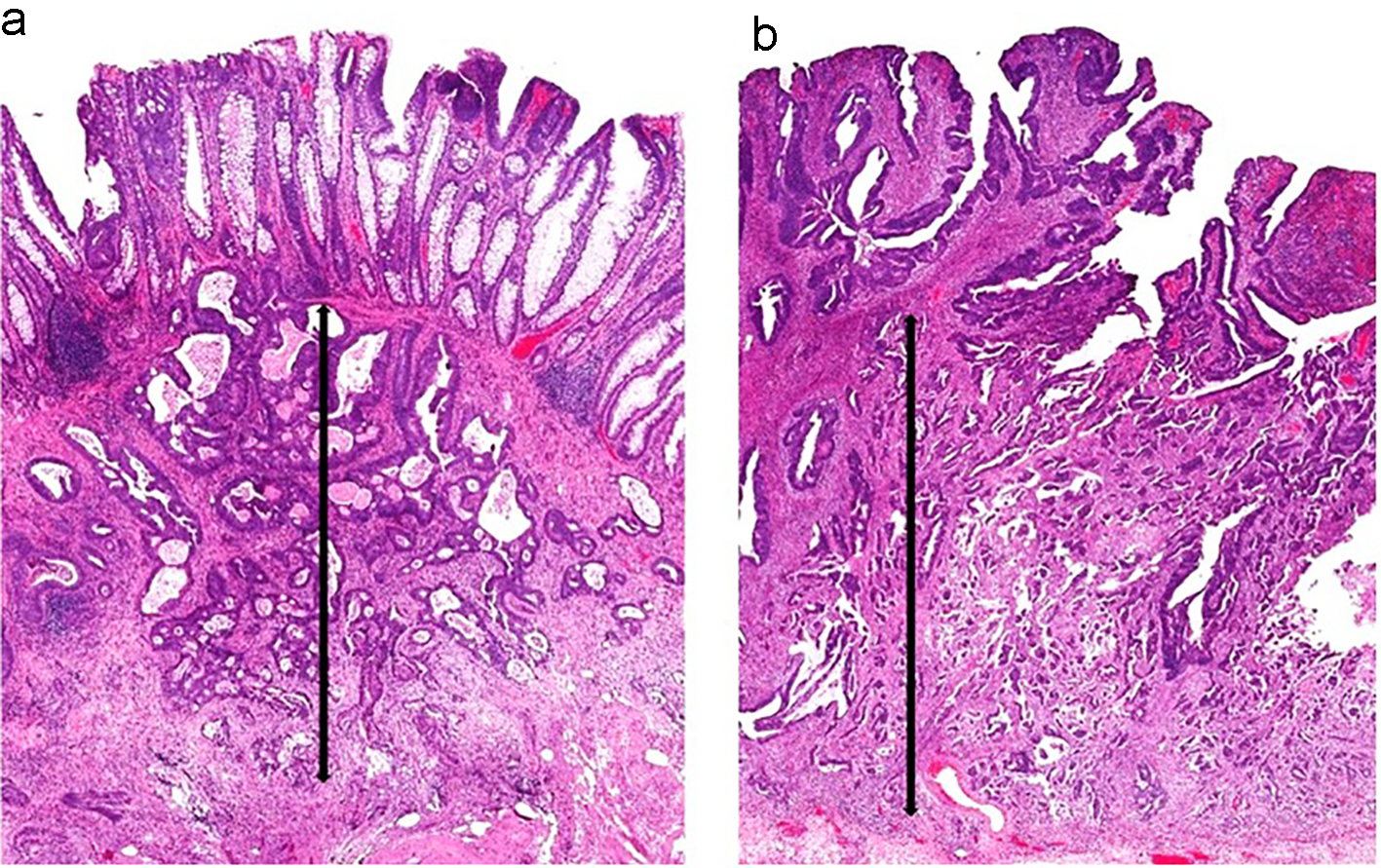 Click for large image | Figure 9. Two sessile polyps and their respective measurements for depth of submucosal invasion. (a) The muscularis mucosae are apparent in this polyp. The depth of submucosal invasion is measured from the muscularis mucosae to the deepest invasive component (black arrow). (b) The muscularis mucosae are obliterated by carcinoma in this polyp. Thus, the submucosal invasion measurement is taken from the most superficial aspect of tumor invasion down to its deepest extension (black arrow). |
A meta-analysis of the literature demonstrates that in both sessile and pedunculated malignant polyps, a depth of submucosal invasion > 1,000 µm was significantly associated with lymph node metastasis (odds ratio (OR) 3.87 (95% confidence interval (CI) 1.50 - 10.00); P = 0.005) [63]. Another meta-analysis study also found that an invasion depth of ≥ 1,000 µm into the submucosa showed a strong increase in relative risk (RR) for lymph node metastasis (RR 5.2 (95% CI 1.8 - 15.4)) [64]. One study with 116 surgically resected pT1 colorectal carcinomas showed that the depth of submucosal invasion > 1,000 µm (P = 0.04) was significantly associated with lymph node metastasis on univariate analysis for both sessile and pedunculated polyps [65]. In a retrospective multi-institutional cross-sectional study of 806 pT1 colorectal cancer cases, the depth of submucosal invasion ≥ 1,000 µm (OR 5.56 (95% CI 2.14 - 19.10)) is an independent predictor of lymph node metastasis for both sessile and pedunculated polyps by multivariate analysis [10]. According to the Japanese Society for Cancer of the Colon and Rectum guidelines 2019 for treatment of colorectal cancer, if the depth of submucosal invasion is ≥ 1,000 µm, intestinal resection with lymph node dissection is recommended [66, 67]. The overall data and guidelines suggest that a depth of submucosal invasion > 1,000 µm is significantly associated with lymph node metastasis for both sessile and pedunculated polyps. Due to the significance of measuring submucosal invasion in malignant polyps, the measurements are best performed by ocular micrometer or by images captured on camera using software.
Differentiation
Tumor differentiation has been shown to be associated with adverse outcomes in colorectal cancer. Three tumor grades have been described for malignant colorectal tumors. Grade 1 is a well-differentiated intestinal-type adenocarcinoma, composed of well-formed glands with open lumina or with more than 95% glandular differentiation. Grade 2 adenocarcinoma is a moderately differentiated intestinal-type adenocarcinoma, which has 50-95% glandular differentiation. Grade 3 adenocarcinoma is a poorly differentiated carcinoma which contains less than 50% glandular differentiation [68] (Fig. 10). Poorly differentiated adenocarcinomas are associated with significantly increased mortality (OR 9.2; P < 0.05) [8]. One meta-analysis of histopathological factors contributing to the risk of lymph node metastasis in early colorectal cancer revealed that poorly differentiated adenocarcinomas, compared with well or moderately differentiated adenocarcinoma, were significantly associated with the incidence of lymph node metastases (OR 5.60 (95% CI 2.90 - 10.82); P < 0.00001). In contrast, well-differentiated adenocarcinomas had a significantly lower risk of lymph node metastases than moderately or poorly differentiated tumors (OR 4.74 (95% CI 3.37 - 6.67); P < 0.00001) [63]. Another meta-analysis also concluded that patients with poorly differentiated adenocarcinoma in malignant colorectal polyps had higher rates of lymph node metastases or hematogenous metastases. Poorly differentiated adenocarcinomas in malignant colorectal polyps also had a significantly higher cancer-related mortality compared to their counterparts [69]. According to the Japanese Society for Cancer of the Colon and Rectum guidelines 2019 for the treatment of colorectal cancer, if there is poorly differentiated adenocarcinoma present in a polyp, intestinal resection with lymph node dissection is recommended [66].
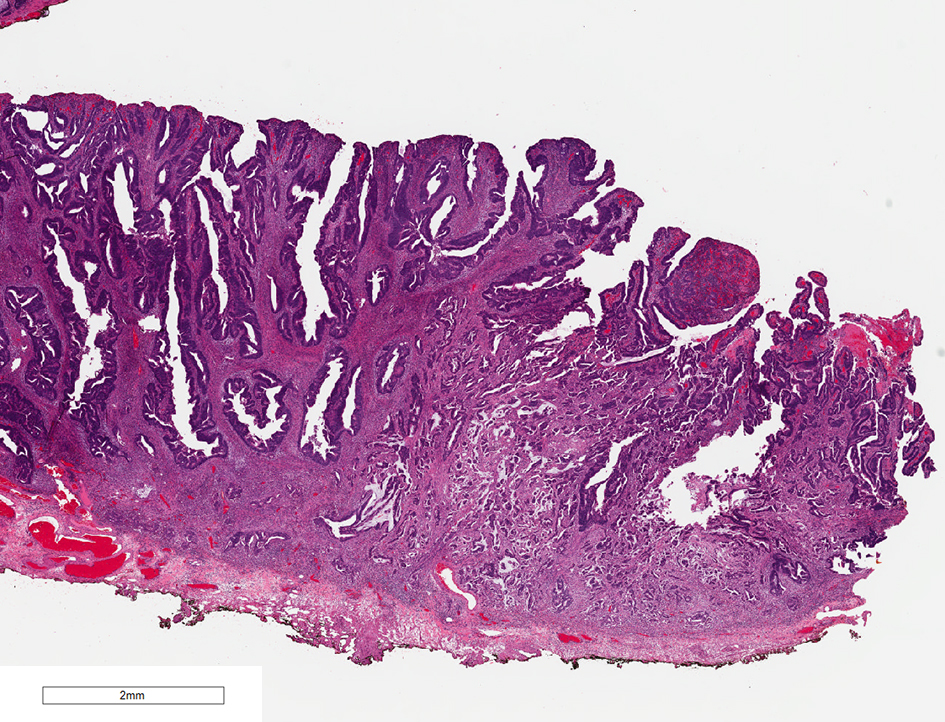 Click for large image | Figure 10. H&E stain of a malignant polyp. Grade 3 adenocarcinoma is a poorly differentiated carcinoma which contains less than 50% glandular differentiation. H&E: hematoxylin and eosin. |
Deep margin status and cutoff value
The distance of invasive adenocarcinoma from the deep resection margin correlates closely with the risk of recurrence. The risk of disease recurrence ranges from 0% to 2% in malignant polyps if the distance of invasive adenocarcinoma from the resection margin is greater than 1 mm. However, when the resection margin is involved, or if the distance from tumor to the resection margin is less than 1 mm, the risk of tumor recurrence ranges between 21% and 33% [70, 71]. Historically, the cutoff for a positive margin was set at 2 mm from the tumor, as studies have shown that the possibility of residual disease or tumor recurrence is low [70, 72-75]. However, current College of American Pathologists colorectal cancer protocol notes that the presence of tumor at or less than 1 mm from the resection margin has an increased risk for an adverse outcome, and therefore has set the cutoff at 1 mm. Malignant polyps with invasive adenocarcinoma at the deep margin or within 1 mm of the closest deep margin (Fig. 11) are considered incomplete resections and need subsequent oncologic resection to reduce the risk for local recurrence.
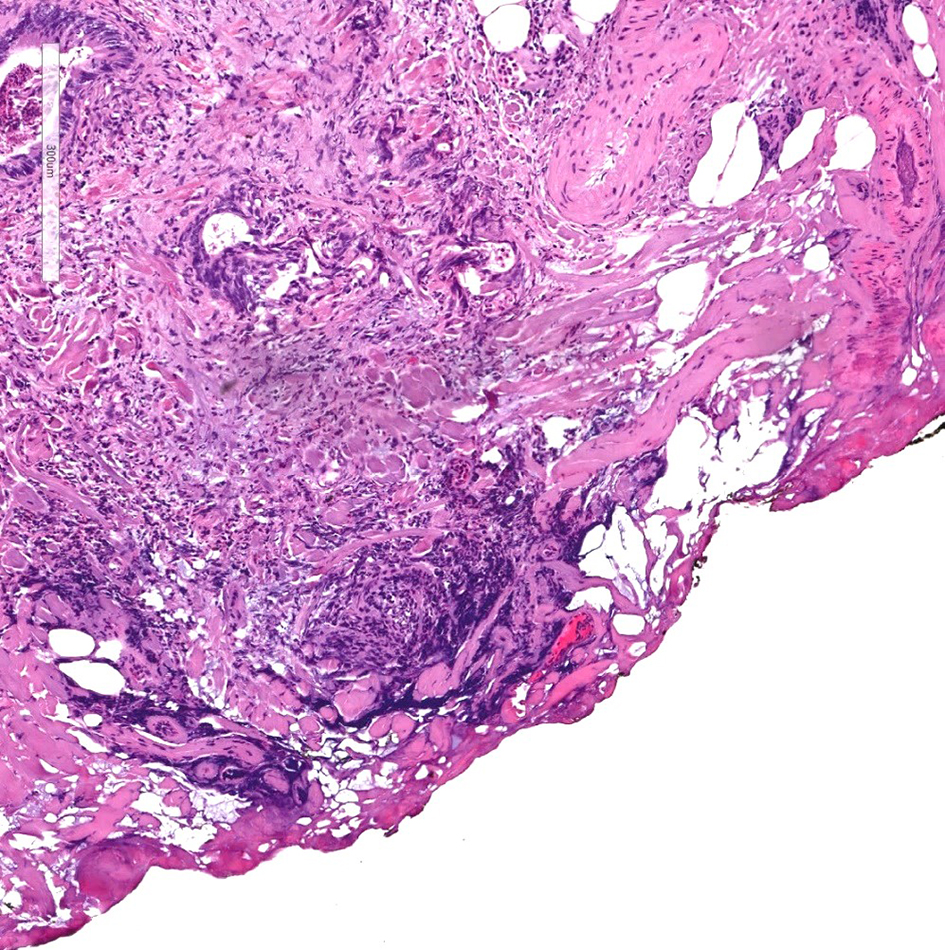 Click for large image | Figure 11. H&E stain of a cauterized polyp margin with tumor. A positive deep margin in a malignant polyp is defined as the presence of invasive adenocarcinoma within 1 mm of the inked or cauterized deep margin. H&E: hematoxylin and eosin. |
Lymphovascular invasion
Lymphovascular invasion has been found to be significantly associated with the risk of lymph node metastasis in many studies [48, 76-79]. Early studies found that lymphatic channels are often present near nests of infiltrating tumors in malignant polyps [68, 80], and that the occurrence of lymphovascular invasion is generally found in malignant polyps with positive resection margins and/or the presence of poorly differentiated tumors [75, 81]. The prevalence of venous invasion in malignant polyps ranges from 3.5% to 39% [82]. Venous invasion is associated with lymphatic invasion, a resection margin of less than 2 mm and/or poor differentiation. Although it is not recommended to routinely perform immunohistochemical staining for the detection of the lymphovascular invasion, stains such as D2-40, CD31, factor VIII, CD34 and ERG can be very helpful for confirming lymphovascular invasion in equivocal cases [83, 84]. Similarly, it is not recommended to routinely perform Verhoeff-Van Gieson (VVG) stain for venous invasion, but VVG stain can be used to confirm venous invasion in equivocal cases (Fig. 12).
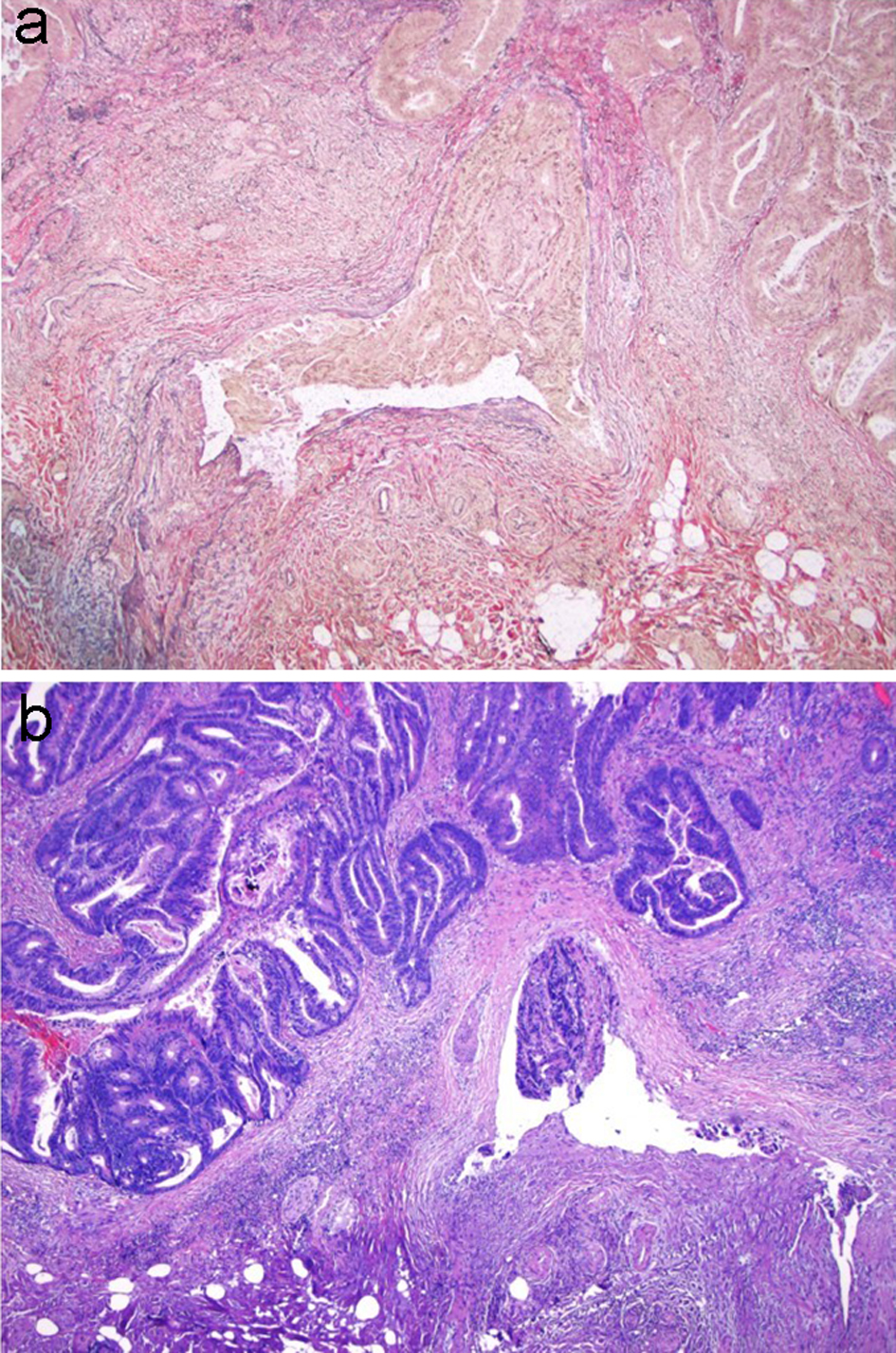 Click for large image | Figure 12. VVG stain is used to evaluate for large vessel invasion. (a) VVG stain reveals venous invasion in this case of invasive adenocarcinoma. (b) H&E stain demonstrates orphan arterioles associated with adjacent venous invasion. VVG: Verhoeff-Van Gieson; H&E: hematoxylin and eosin. |
Tumor budding
Tumor budding is defined as the presence of single tumor cells or small clusters of less than five tumor cells at the advancing front of the tumor (Fig. 13). Per an International Tumor Budding Consensus Conference (ITBCC) in 2016 [85], tumor budding counts should be done on hematoxylin and eosin (H&E) sections, and tumor budding should be reported by selecting a “hotspot” which is chosen after reviewing all available slides with invasive tumor. The total number of tumor budding should be reported in an area measuring 0.785 mm2, which corresponds to a × 20 field in some microscopes. For a specific microscope, the tumor bud count per 0.785 mm2 can be calculated by dividing the bud count on a × 20 field by the normalization factor. Both the total number of buds and a three-tier score (based on a 0.785 mm2 field area) should be reported: low (0 - 4 buds), intermediate (5 - 9 buds) and high (10 or more buds). Studies have shown that high tumor budding is a significant risk factor for lymph node metastasis in adenocarcinomas arising from polyps [64, 86-90]. One meta-analysis of histopathological factors influencing the incidence of lymph node metastasis in early colorectal cancer also revealed that tumor budding is significantly associated with the risk of lymph node metastasis (OR 7.74 (95% CI 4.47 - 13.39); P < 0.001) [63]. The Japanese Society for Cancer of the Colon and Rectum guidelines 2019 for the treatment of colorectal cancer recommends intestinal resection with lymph node if there are more than five tumor buds at the site of deepest invasion [66, 67].
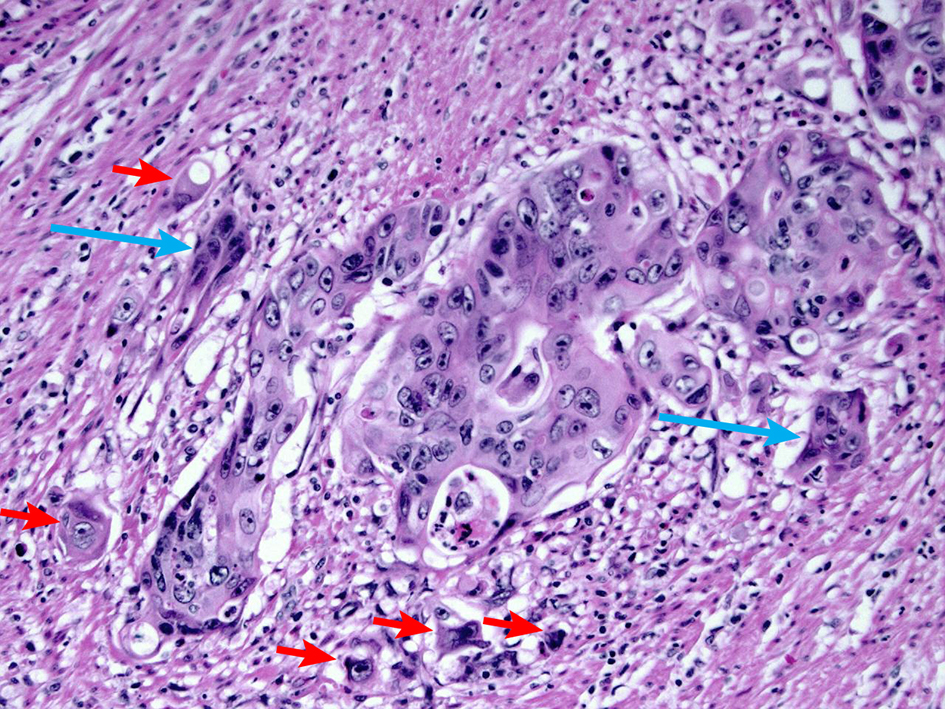 Click for large image | Figure 13. H&E stain demonstrating tumor budding. Tumor budding is defined as the presence of single tumor cells or small clusters of less than five tumor cells at the advancing front of the tumor (short arrows). Cancer clusters of more than five tumor cells showing invasion into the stroma without gland formation are defined as poorly differentiated clusters (long arrow). A high level of tumor budding is associated with nodal metastasis in early colorectal adenocarcinoma. A high level of poorly differentiated clusters is associated with a high recurrence rate. H&E: hematoxylin and eosin. |
Poorly differentiated clusters (PDCs)
PDCs are defined as clusters of more than five tumor cells that have no gland formation and show invasion into the stroma (Fig. 13). In one study of advanced colorectal cancer, PDCs were classified into three grades. Grade 1 PDCs are tumors with < 10 clusters in a microscopic field of a × 4 objective lens. Grade 2 PDCs are tumors with ≥ 10 clusters in a microscopic field of a × 4 objective lens. Grade 3 PDCs are tumors with poorly differentiated tumor clusters fully occupying the microscopic field of a × 40 objective lens. The authors found that patients with tumors showing grade 1 PDCs had a very favorable prognosis, with a 99.3% cancer-related 5-year survival rate. However, the 5-year survival rate was 86.0% for tumors with grade 2 PDCs and 68.9% for tumors with grade 3 PDCs (P < 0.0001 in each group). Multivariate analysis revealed that the grades of PDCs were an independent prognosticator [91]. A different study also classified colorectal cancer into three grades according to their respective PDCs, but used different cutoff numbers: grade 1: < 5, grade 2: 5 - 9 and grade 3: ≥ 10 PDCs. This study showed that different tumor PDC grades were significantly associated with recurrence-free survival rates. The recurrence-free survival rates were 91.6% for grade 1, 75.4% for grade 2 and 59.6% for grade 3 (P < 0.0001) [92]. Another study found that the PDC-based grading system had a higher weighted κ coefficient for interobserver variability (five observers) compared to conventional grading systems, which are based on degrees of tumor differentiation [93]. Furthermore, a study suggested that grading of PDCs could be introduced in routine histopathological reports of colorectal cancer and be used for therapeutic decision making [94]. However, the significance of PDCs may be overlapped with poor differentiation of the tumors. Additional studies are needed to validate the significance of PDCs, independent of other adverse factors.
Micropapillary features
Colorectal adenocarcinomas that show micropapillary features are associated with lymph node dissection metastases. A micropapillary pattern is characterized by small, tight clusters of tumor cells with cleft-like spaces (Fig. 14). In a study of 178 cases of colorectal carcinoma, a micropapillary component was seen in 34 (19.1%) cases. Lymph node metastases were found in 61 of 144 (42.4%) cases that did not have a micropapillary component and in 25 of 34 (73.5%) carcinomas that contained a micropapillary component (P = 0.001). Similarly, the rate of lymphovascular invasion was significantly lower in carcinomas without a micropapillary component (20.1%) and more frequently identified in carcinomas with a micropapillary component (41.2%; P < 0.05) [95]. Another study showed that colorectal carcinomas with micropapillary architecture had higher frequency of infiltrative features, higher rates of lymphovascular and perineural invasion, greater depths of invasion, and more positive lymph nodes compared to conventional adenocarcinomas [96]. Furthermore, Pyo et al reported that colorectal carcinomas with micropapillary pattern had higher rates of vascular and lymphatic invasion, higher metastatic lymph node ratios, and higher pT stages compared to colorectal carcinomas without a micropapillary pattern. The presence of a micropapillary pattern in colorectal carcinomas was significantly correlated with worse overall survival (P = 0.010) [97]. Altogether, these studies suggest that colorectal carcinomas with micropapillary pattern have a worse prognosis. However, the prognostic significance of this feature in malignant polyps is unknown, and additional intra- and inter-observer agreement studies are needed in the setting of malignant polyps.
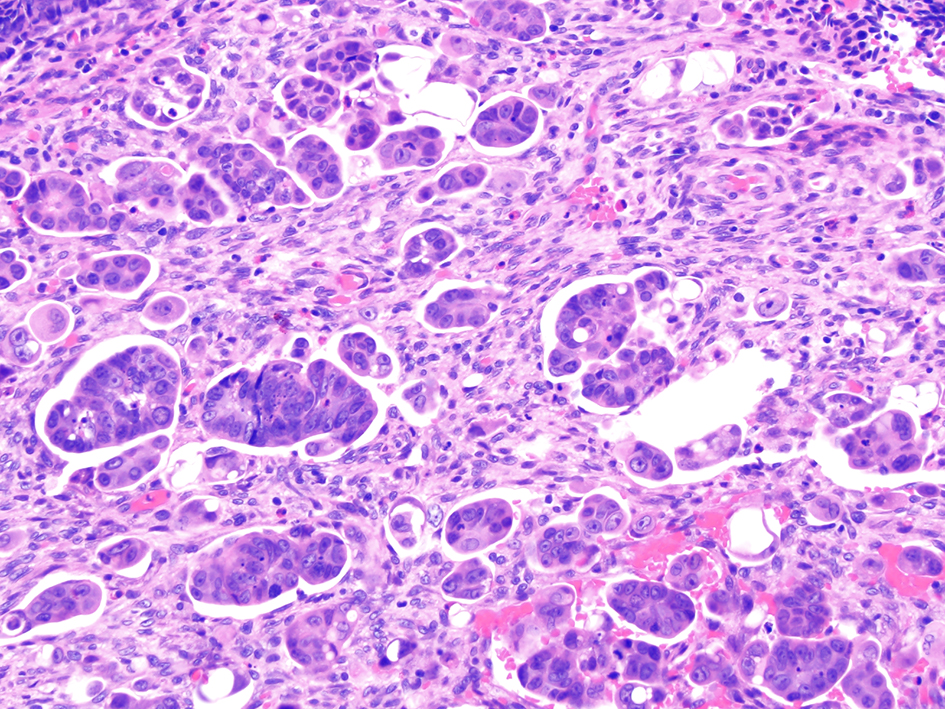 Click for large image | Figure 14. Invasive colorectal adenocarcinoma demonstrating a micropapillary growth pattern. A micropapillary pattern is characterized by small and tight clusters of tumor cells with surrounding cleft-like spaces. This histologic feature is associated with lymphovascular invasion and nodal metastasis. |
Desmoplasia type
Desmoplastic reactions have been classified into three types based on the products of activated fibroblasts: immature, intermediate and mature type [98]. An immature desmoplastic reaction is defined as the presence of myxoid stroma greater than a microscopic field of a × 40 objective lens within the extramural tumor front. If the myxoid stroma does not cover a × 40 field and the extramural tumor front has keloid-like collagen fibers, then the stroma has an intermediate desmoplastic reaction (Fig. 15). A mature desmoplastic reaction shows fine collagen fibers in the absence of both myxoid stroma (greater than a microscopic field of a × 40 objective lens) and keloid-like collagen. It has been reported that desmoplastic reaction was the strongest prognostic indicator of disease-specific survival in stage II colorectal cancer among the risk factors examined, including tumor budding, PDCs and Crohn-like lymphoid reaction [99]. In addition, categorization of desmoplastic reaction was significantly associated with tumor location, pT and pN stages, tumor differentiation, venous invasion, tumor budding and Crohn-like lymphoid reaction (P ≤ 0.0001 - 0.008) in stage II and III colorectal cancer patients. Immature desmoplastic reactions were found to have high incidences of recurrence in the liver, lung, lymph nodes, peritoneum and locoregional areas (P ≤ 0.0001 - 0.002). The 5-year disease-free survival rate was highest in the mature desmoplastic reaction group (87%), followed by the intermediate group (72%) and the immature group (49%; P < 0.0001) [100]. Another study found that the 5-year relapse-free survival rate was highest in the mature desmoplastic reaction group (85.7%), followed by the intermediate desmoplastic reaction (77.3%) and immature (50.4%) desmoplastic reaction groups [98]. These studies suggest that desmoplastic reaction is a strong prognostic indicator and the immature desmoplastic reaction is associated with a worse prognosis compared to mature and intermediate desmoplastic reactions. The prognostic significance of this feature in malignant polyps and the degrees of intra- and inter-observer agreement have not yet been studied.
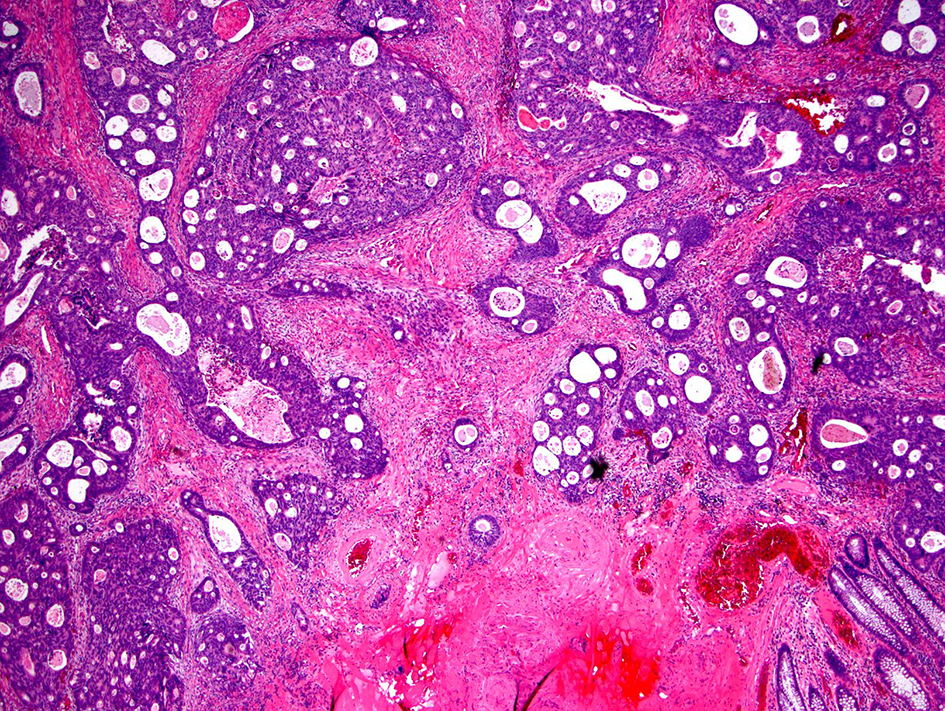 Click for large image | Figure 15. Invasive colorectal adenocarcinoma with an intermediate desmoplastic stromal reaction. Intermediate desmoplasia is characterized by the presence keloid-like collagen and myxoid stroma less than a microscopic field of a × 40 objective lens. |
| Other Special Issues | ▴Top |
Intramucosal adenocarcinoma
Intramucosal carcinoma is defined as carcinoma invading into the lamina propria without extension through the muscularis mucosae. It is also called carcinoma in situ (pTis) (Fig. 16). Lymphatic channels are normally present in the superficial submucosa and within the muscularis mucosae in the colon [101]. There is a near absence of lymphatics within the colonic mucosa. Therefore, intramucosal carcinoma in the colon is associated with negligible potential for lymph node metastasis. Intramucosal carcinoma in a colonic polyp with negative margins usually does not require further surgery [68]. A subset of intramucosal adenocarcinoma can be poorly differentiated and limited experience suggests that a conservative approach without oncological resection may be adequate if the entire polyp is removed and can be evaluated for depth of invasion [102]. However, additional larger studies are needed to truly determine the risk of metastasis of poorly differentiated intramucosal adenocarcinomas of the colorectum. The World Health Organization (WHO) recommends using the term “high-grade dysplasia” instead of intramucosal carcinoma in the colon. Although the terminology of intramucosal carcinoma is not recommended for use in completely resected polyps/lesions, some pathologists may use this term when the intramucosal adenocarcinoma is poorly differentiated and the polyp is completely removed by polypectomy. Additionally, in biopsies or partially resected specimens, the term “intramucosal adenocarcinoma” or “at least intramucosal adenocarcinoma” may be used to indicate the “malignant” and uncertain behavior of the lesion.
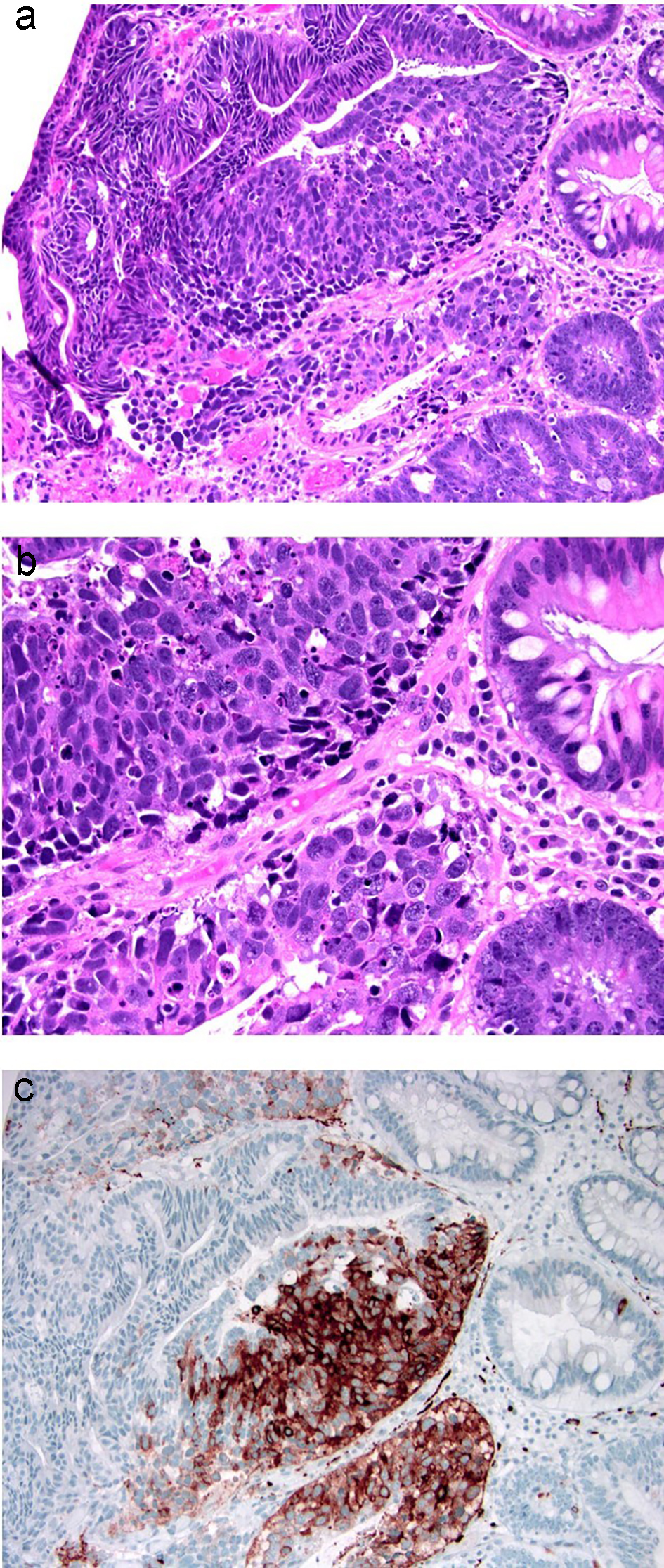 Click for large image | Figure 16. Colonic adenoma with focal intramucosal high-grade neuroendocrine carcinoma. (a, b) Tubular adenoma with a single cluster of cells exhibiting extremely high nuclear to cytoplasmic ratio and brisk apoptosis, breaching the basement membrane of the glands. (c) This focus is strongly positive for synaptophysin. Ki-67 immunolabeling showed a high proliferative index of up to 90% (not depicted). |
Intramucosal signet-ring cell carcinoma
Primary signet-ring cell carcinomas of the colon and rectum were first described by Laufman and Saphir in 1951 [103]. Signet-ring cell carcinomas are characterized by tumor cells with abundant intracytoplasmic mucin and peripherally placed nuclei. The prevalence of signet-ring cell carcinoma is less than 3% of colorectal cancers [104, 105], and they are usually diagnosed at an advanced stage. Intramucosal signet-ring cell carcinoma in the colorectum is rare [102, 106-112]. It has been reported that colorectal signet-ring cell carcinoma may have an adenomatous component [106, 109]. Tumors showing signet-ring cells without accompanying adenoma or other types of cancer cells were also reported [113]. Due to these conflicting reports, it is not clear whether signet-ring cell carcinoma arises from a pre-existing adenomatous polyp or via a de novo pathway [114]. It has been suggested that intramucosal signet-ring cell carcinoma in the colorectum can be managed endoscopically [102]; however, larger studies are needed. According to the Japanese Society for Cancer of the Colon and Rectum guidelines 2019 for the treatment of colorectal cancer, after endoscopic resection of a pT1 colorectal cancer, intestinal resection with lymph node dissection is recommended as an additional treatment if signet-ring cell carcinoma is present [66].
Intramucosal neuroendocrine carcinoma
Intramucosal neuroendocrine carcinoma is extremely rare in the colon. One case of intramucosal neuroendocrine carcinoma in the rectosigmoid colon was detected by flexible sigmoidoscopy as a 40 mm sessile polyp [115]. Histologically, it showed fragments of tubulovillous adenoma with extensive high-grade dysplasia, and a single 1 mm focus of intramucosal neuroendocrine carcinoma was identified. The tumor cells were strongly and diffusely positive for both synaptophysin and chromogranin A, with a Ki-67 proliferation index of nearly 100%. Both components of tubulovillous adenoma and intramucosal neuroendocrine carcinoma had intact expression of mismatch repair proteins MLH1, PMS2, MSH2 and MSH6 by immunohistochemistry. The patient was alive 1 year after the polypectomy. Another similar case was reported; however, details of the treatment were not provided [102]. It is unknown whether polypectomy is an adequate treatment for intramucosal neuroendocrine carcinoma. Gaffey et al reported an intramucosal neuroendocrine carcinoma removed by colectomy. No nodal or distant metastases were identified at the time of colectomy; however, no follow-up information for this patient was reported [116]. Very little is known about colorectal intramucosal neuroendocrine carcinomas and their biological behavior.
Gut-associated lymphoid tissue (GALT) or “dome” carcinoma of the colon
GALT/dome-type carcinomas of the colon are thought to arise from M cells within the lymphoglandular complex (LGC) of the intestine. M cells are specialized columnar epithelial cells (microfold, glycocalyx-free “M cells”) that overlie the lymphoid follicles of the gut and form a single cell layer. M cells function by translocating antigens and pathogens from the gut lumen to the underlying lymphoid population. Epithelium of the LGC may herniate into the submucosa, forming so-called “ectopic colonic mucosa”, more commonly seen in patients with inflammatory bowel disease [117]. Conventional colonic adenomatous dysplasia may extend along the mucosa and into invaginations of the LGC within the submucosa.
Dome carcinoma was first described in a patient with ulcerative colitis with the coined term “GALT carcinoma”. Later, the term “dome-type carcinoma” was used by De Petris [118]. Endoscopically, dome-type carcinomas are elevated, dome-like, or plaque-like/sessile lesions. Histologically, dome-like carcinomas comprise of dilated, malignant/dysplastic glands lined by columnar epithelial cells with eosinophilic cytoplasm and a background of prominent lymphoid tissue [118]. GALT/dome-type carcinoma affects both genders, with wide ranges in age of presentation (36 - 77 years) and tumor size (5 - 30 mm) [119]. All GALT/dome-type carcinomas have been found to be microsatellite stable. Almost all GALT/dome-type carcinomas are limited to the submucosa, and recurrences and metastases to lymph nodes or distant sites have not been documented. Limited experience suggests that adequate local excision is sufficient treatment for GALT/dome-type carcinomas.
GALT/dome-type carcinoma should be distinguished from LGC involvement by conventional adenoma. The presence of dilated glands lined by eosinophilic columnar cells and closely intermingled with lymphoid tissue favors GALT/dome-type carcinoma. MSI-H colorectal adenocarcinoma may have prominent lymphoid tissue, mimicking GALT/dome-like carcinoma. Conventional dysplasia will exhibit more cytologic atypia with vesicular nuclei and prominent nucleoli. Conventional dysplasia will also not have the eosinophilic cytoplasm or cystic dilatations seen in GALT/dome-type carcinomas. An MSI-H status will distinguish the two entities from each other.
Colonic submucosally invasive adenocarcinoma involving submucosal LGCs
Colonic glandular neoplasia can involve submucosal LGCs and pose diagnostic challenges. A diagnostic pitfall would be to diagnose a colonic adenomatous polyp involving submucosal LGCs as an invasive adenocarcinoma. Histologic features supporting involvement of LGCs by an adenoma include continuity of glands with the overlying surface adenoma, presence of lymphoid tissue completely surrounding dysplasia (Fig. 17) and confinement of dysplastic glands in the LGCs to the lamina propria [120]. Some cases may show fibrosis associated with the dysplastic glands within submucosal lymphoid tissue. The fibrosis seen in these cases are more layered, do not show an edematous appearance and should not be in direct contact with the dysplastic glands [120]. In contrast, invasive colonic adenocarcinomas involving submucosal LGCs often extend beyond the lymphoid aggregates into submucosa, and thus are often only partially surrounded by lymphoid aggregates [120]. In colonic glandular neoplastic lesions closely associated with submucosal LGCs, identification of single cells/small clusters, abortive/irregular glands, solid tumor growth, desmoplasia in direct contact with malignant glands, intraluminal necrosis and/or lymphovascular invasion can help diagnose submucosally invasive adenocarcinoma involving LGCs [120].
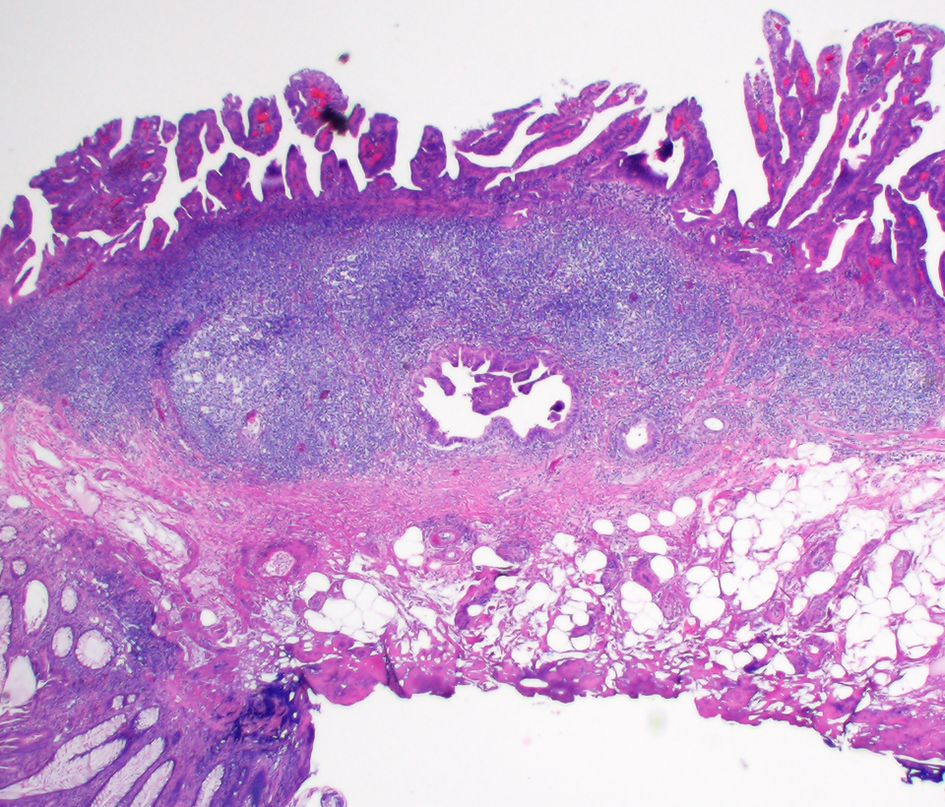 Click for large image | Figure 17. Colonic glandular neoplasia can involve submucosal lymphoglandular complexes (LGCs) and pose diagnostic challenges. In this case, the focus of submucosally located dysplastic glands is entirely surrounded by lymphoid aggregates. This focus is also in continuity with the overlying surface adenoma on additional levels (not depicted). |
Composite intestinal adenoma-microcarcinoid
A composite intestinal adenoma-microcarcinoid is a much rarer colorectal lesion than a microcarcinoid. It is comprised of conventional adenomatous components intermingled with smaller microcarcinoids [121] (Fig. 18). A total of 52 cases have been reported in the literature [121-127]. In brief, composite intestinal adenoma-microcarcinoids occur in a wide age range, from 28 to 82 years of age and have a nearly equal distribution between men (n = 28) and women (n = 24) (male/female = 1.17:1). The size of the polyp ranges from 5 to 127 mm in greatest dimension. Histology of the glandular component may show that of a tubular adenoma or tubulovillous adenoma, and the glandular component is rarely composed of an intramucosal adenocarcinoma or invasive adenocarcinoma [122]. The microcarcinoid component of these lesions range in size from 0.5 to 20 mm and are commonly composed of well-differentiated neuroendocrine cells arranged in small clusters, glands or cords. The neuroendocrine component is often situated at the base of the lamina propria, without submucosal involvement. There have been no cases to date that showed significant proliferative activity in the microcarcinoid components by Ki-67 immunolabeling. Notably, the neuroendocrine component can also form cellular aggregates resembling squamous morules [125, 127]. The presence of focal “desmoplastic” stromal change, along with individual or small nests of infiltrating cells, can be misdiagnosed as invasive adenocarcinoma in a polypectomy specimen and lead to unnecessary surgery [122]. Recognition of the typical well-differentiated neuroendocrine features, supplemented with immunohistochemistry for chromogranin and/or synaptophysin, is critical for the diagnosis. Complete removal of composite intestinal adenoma-microcarcinoids is considered curative, with one exceptional case showing metastatic high-grade neuroendocrine carcinoma involving one lymph node [123].
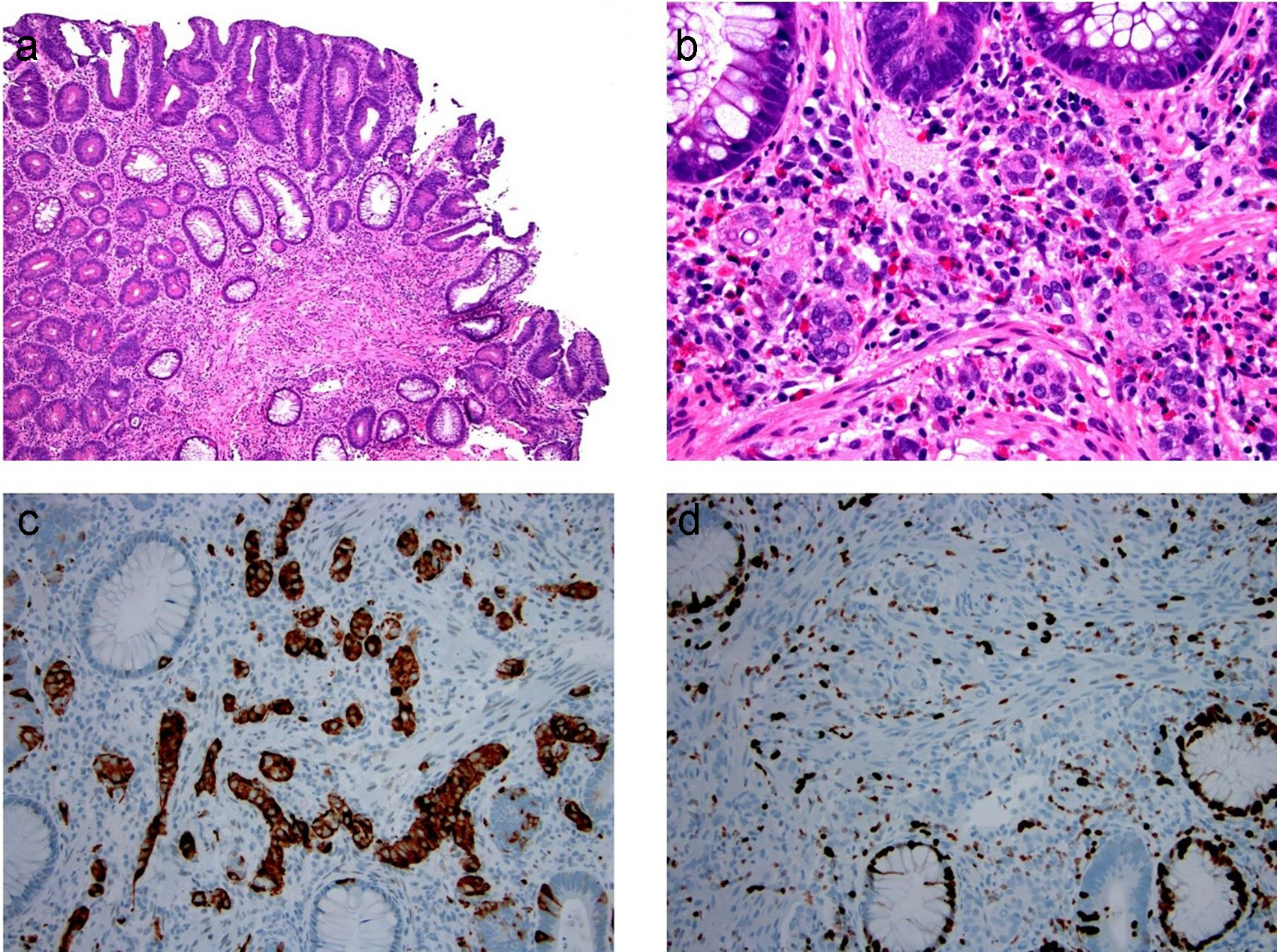 Click for large image | Figure 18. Composite intestinal adenoma-microcarcinoid consists of colonic adenoma and microcarcinoid. (a, b) Microcarcinoids in these lesions are more commonly well-differentiated neuroendocrine cells arranged in small clusters, glands, or cords in the basal lamina propria. (c, d) These cells are positive for synaptophysin (c) and have low Ki-67 immunolabeling (d). |
Changes related with tattoo and Orise gel
Endoscopic tattooing is a useful method for surgical/endoscopic localization of small lesions within the colon. It is commonly used for preoperative localization of lesions [128-130], but may also be used to identify the sites of locally advanced rectal cancer before neoadjuvant chemoradiation therapy. Endoscopic tattooing is often regarded as a minimally invasive procedure without risks for major complications. However, few complications of endoscopic tattooing have been documented in the literature, especially with the use of India ink. Tattooing the colon with India ink can cause fat necrosis, inflammatory pseudotumors [131], idiopathic inflammatory bowel disease [132] and peritoneal tumor metastases [133]. Spot (carbon black) is a newer ink that has been approved by the US Food and Drug Administration (FDA) for endoscopic tattooing. Spot is composed of a sterile suspension of carbon particles, and it has been reported to remain in tissue for up to 1 year after injection [134]. Endoscopic tattooing of tumors with Spot can also inoculate tumor cells into the peri-colonic adipose tissue through a transmural tract [135]. Circumferential injection of tattoo ink should be performed by the endoscopist in order to avoid the possibility of pushing tumor cells into benign tissue with the injection needle [135-137].
Colorectal polyps are often removed endoscopically by first injecting a submucosal lifting agent to fully visualize and completely remove the polyps. In the past, saline has been the most commonly used solution for this purpose. Orise is a new lifting agent which, unlike other submucosal lifting agents, has the advantage of immediate availability without requiring media preparation during the procedure. Histologically, Orise at the previous injection site has been reported to show deposition of pink, hyalinized, amorphous, ribbon-like/globular material in the submucosa with associated foreign body giant cell reaction and fibrosis, even in areas beyond the injection site. These histologic features are similar in morphology to amyloid; however, Congo red stain will be negative for Orise [138, 139]. Compared to amyloid, the changes associated with Orise are more cellular, denser and more deeply eosinophilic. The histologic changes caused by Orise deposits will also show overlap with hyalinized pulse granulomata because both may have giant cell reactions associated with eosinophilic material. The eosinophilic material in hyalinized pulse granulomata will not show staining on Congo red and periodic acid-Schiff (PAS) stains. In contrast, Orise deposits are positive for PAS with diastase (PAS/D) [138]. The Orise deposits can also histologically mimic mucin in specimens that are immediately resected after Orise injection (Fig. 19), and these features may cause diagnostic challenges and lead to misdiagnosis of mucinous adenocarcinoma. Awareness of Orise injections performed during the procedure and the visualization of uniform gray-pink material in the submucosa without associated stromal or inflammatory responses are helpful clues to avoid the diagnostic pitfall. In doubtful cases, a mucin stain such as PAS/D stain will prove useful, as Orise is PAS/D negative in the polypectomy specimen removed immediately after injection [140].
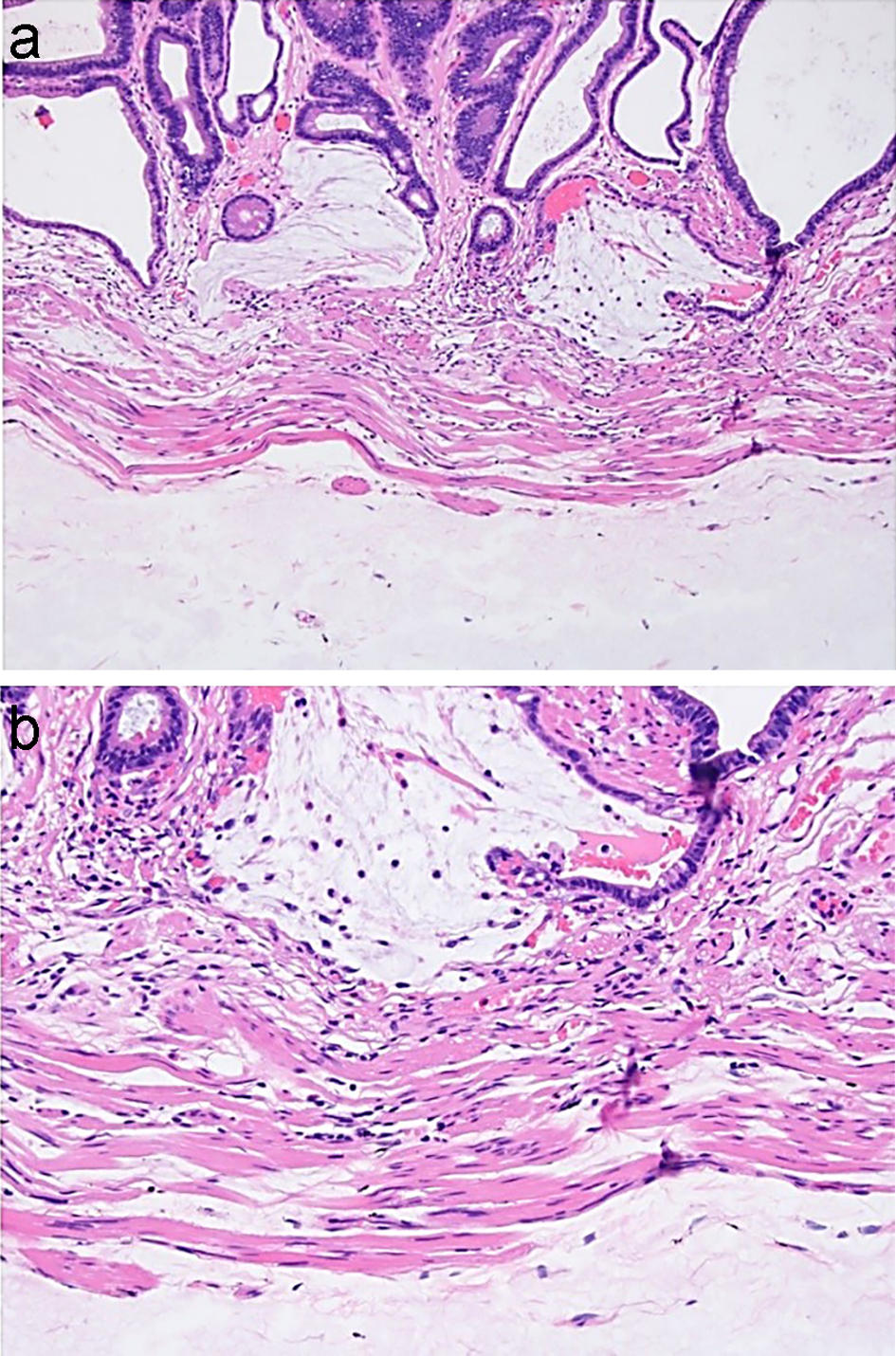 Click for large image | Figure 19. Orise in the polypectomy specimen removed immediately after Orise injection may closely mimic mucin. (a, b) Mucin associated with crypt rupture is seen above the muscularis mucosae. Orise gel can be seen below the muscularis mucosae. |
| Conclusion | ▴Top |
Colorectal malignant polyps are cancerous polyps that consist of tumor cells invading through the muscularis mucosae into the underlying submucosa. The potential for lymph node metastasis in these polyps ranges from 8.5% to 16.1%. Correctly diagnosing and reporting adverse histologic features of malignant polyps are critical for clinical teams to make subsequent treatment decisions and establish appropriate surveillance schedules after local excision of these polyps. There are an increasing number of identifiable adverse histologic features including Haggitt level 4, depth of invasion >1,000 µm, poor tumor differentiation, positive deep margin (tumor at or within 1 mm of the deep margin), lymphovascular invasion, tumor budding and micropapillary features which should be reported in the pathology report in cases of malignant polyp. There are recent advancements in endoscopic treatment techniques including more use of ESD. As gastroenterologists and pathologists, we need to be aware of all the factors and different treatment options in order to provide patients with the best care after local excision of their malignant polyps. Proper endoscopic/macroscopic and microscopic pathologic assessments of polyps are required in an era of precision medicine.
Acknowledgments
None to declare.
Financial Disclosure
The authors declare that they do not have a financial relationship with any commercial entity that has an interest in the subject of this manuscript.
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Author Contributions
All authors participated in review. They were involved in writing and revising the article prior to submission.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, Cercek A, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70(3):145-164.
doi pubmed - Goto H, Oda Y, Murakami Y, Tanaka T, Hasuda K, Goto S, Sasaki Y, et al. Proportion of de novo cancers among colorectal cancers in Japan. Gastroenterology. 2006;131(1):40-46.
doi pubmed - Robert ME. The malignant colon polyp: diagnosis and therapeutic recommendations. Clin Gastroenterol Hepatol. 2007;5(6):662-667.
doi pubmed - Coverlizza S, Risio M, Ferrari A, Fenoglio-Preiser CM, Rossini FP. Colorectal adenomas containing invasive carcinoma. Pathologic assessment of lymph node metastatic potential. Cancer. 1989;64(9):1937-1947.
doi - Tateishi Y, Nakanishi Y, Taniguchi H, Shimoda T, Umemura S. Pathological prognostic factors predicting lymph node metastasis in submucosal invasive (T1) colorectal carcinoma. Mod Pathol. 2010;23(8):1068-1072.
doi pubmed - Aarons CB, Shanmugan S, Bleier JI. Management of malignant colon polyps: current status and controversies. World J Gastroenterol. 2014;20(43):16178-16183.
doi pubmed - Park YJ, Kim WH, Paeng SS, Park JG. Histoclinical analysis of early colorectal cancer. World J Surg. 2000;24(9):1029-1035.
doi pubmed - Hassan C, Zullo A, Risio M, Rossini FP, Morini S. Histologic risk factors and clinical outcome in colorectal malignant polyp: a pooled-data analysis. Dis Colon Rectum. 2005;48(8):1588-1596.
doi pubmed - Boenicke L, Fein M, Sailer M, Isbert C, Germer CT, Thalheimer A. The concurrence of histologically positive resection margins and sessile morphology is an important risk factor for lymph node metastasis after complete endoscopic removal of malignant colorectal polyps. Int J Colorectal Dis. 2010;25(4):433-438.
doi pubmed - Kawachi H, Eishi Y, Ueno H, Nemoto T, Fujimori T, Iwashita A, Ajioka Y, et al. A three-tier classification system based on the depth of submucosal invasion and budding/sprouting can improve the treatment strategy for T1 colorectal cancer: a retrospective multicenter study. Mod Pathol. 2015;28(6):872-879.
doi pubmed - Arends MJ. Pathways of colorectal carcinogenesis. Appl Immunohistochem Mol Morphol. 2013;21(2):97-102.
- Nguyen HT, Duong HQ. The molecular characteristics of colorectal cancer: Implications for diagnosis and therapy. Oncol Lett. 2018;16(1):9-18.
doi - Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396(6712):643-649.
doi pubmed - Markowitz SD, Bertagnolli MM. Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med. 2009;361(25):2449-2460.
doi pubmed - Grady WM, Pritchard CC. Molecular alterations and biomarkers in colorectal cancer. Toxicol Pathol. 2014;42(1):124-139.
doi pubmed - Thompson SL, Bakhoum SF, Compton DA. Mechanisms of chromosomal instability. Curr Biol. 2010;20(6):R285-295.
doi pubmed - Tsang AH, Cheng KH, Wong AS, Ng SS, Ma BB, Chan CM, Tsui NB, et al. Current and future molecular diagnostics in colorectal cancer and colorectal adenoma. World J Gastroenterol. 2014;20(14):3847-3857.
doi pubmed - Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61(5):759-767.
doi - Pino MS, Chung DC. The chromosomal instability pathway in colon cancer. Gastroenterology. 2010;138(6):2059-2072.
doi pubmed - Tanaka T. Colorectal carcinogenesis: Review of human and experimental animal studies. J Carcinog. 2009;8:5.
doi pubmed - Thiagalingam S, Lengauer C, Leach FS, Schutte M, Hahn SA, Overhauser J, Willson JK, et al. Evaluation of candidate tumour suppressor genes on chromosome 18 in colorectal cancers. Nat Genet. 1996;13(3):343-346.
doi pubmed - Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med. 2003;348(10):919-932.
doi pubmed - Ellegren H. Microsatellites: simple sequences with complex evolution. Nat Rev Genet. 2004;5(6):435-445.
doi pubmed - Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993;260(5109):816-819.
doi pubmed - Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, Meltzer SJ, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58(22):5248-5257.
- Pagin A, Zerimech F, Leclerc J, Wacrenier A, Lejeune S, Descarpentries C, Escande F, et al. Evaluation of a new panel of six mononucleotide repeat markers for the detection of DNA mismatch repair-deficient tumours. Br J Cancer. 2013;108(10):2079-2087.
doi pubmed - Findeisen P, Kloor M, Merx S, Sutter C, Woerner SM, Dostmann N, Benner A, et al. T25 repeat in the 3' untranslated region of the CASP2 gene: a sensitive and specific marker for microsatellite instability in colorectal cancer. Cancer Res. 2005;65(18):8072-8078.
doi pubmed - Abdel-Rahman WM, Peltomaki P. Molecular basis and diagnostics of hereditary colorectal cancers. Ann Med. 2004;36(5):379-388.
doi pubmed - Kheirelseid EA, Miller N, Chang KH, Curran C, Hennessey E, Sheehan M, Kerin MJ. Mismatch repair protein expression in colorectal cancer. J Gastrointest Oncol. 2013;4(4):397-408.
- Pal T, Permuth-Wey J, Sellers TA. A review of the clinical relevance of mismatch-repair deficiency in ovarian cancer. Cancer. 2008;113(4):733-742.
doi pubmed - Peltomaki P. Lynch syndrome genes. Fam Cancer. 2005;4(3):227-232.
doi pubmed - Rosty C, Hewett DG, Brown IS, Leggett BA, Whitehall VL. Serrated polyps of the large intestine: current understanding of diagnosis, pathogenesis, and clinical management. J Gastroenterol. 2013;48(3):287-302.
doi pubmed - O'Brien MJ, Zhao Q, Yang S. Colorectal serrated pathway cancers and precursors. Histopathology. 2015;66(1):49-65.
doi pubmed - Bettington M, Walker N, Rosty C, Brown I, Clouston A, McKeone D, Pearson SA, et al. Clinicopathological and molecular features of sessile serrated adenomas with dysplasia or carcinoma. Gut. 2017;66(1):97-106.
doi pubmed - Liu C, Walker NI, Leggett BA, Whitehall VL, Bettington ML, Rosty C. Sessile serrated adenomas with dysplasia: morphological patterns and correlations with MLH1 immunohistochemistry. Mod Pathol. 2017;30(12):1728-1738.
doi pubmed - Murakami T, Akazawa Y, Yatagai N, Hiromoto T, Sasahara N, Saito T, Sakamoto N, et al. Molecular characterization of sessile serrated adenoma/polyps with dysplasia/carcinoma based on immunohistochemistry, next-generation sequencing, and microsatellite instability testing: a case series study. Diagn Pathol. 2018;13(1):88.
doi pubmed - Bettington ML, Walker NI, Rosty C, Brown IS, Clouston AD, McKeone DM, Pearson SA, et al. A clinicopathological and molecular analysis of 200 traditional serrated adenomas. Mod Pathol. 2015;28(3):414-427.
doi pubmed - Borowsky J, Dumenil T, Bettington M, Pearson SA, Bond C, Fennell L, Liu C, et al. The role of APC in WNT pathway activation in serrated neoplasia. Mod Pathol. 2018;31(3):495-504.
doi pubmed - Hashimoto T, Yamashita S, Yoshida H, Taniguchi H, Ushijima T, Yamada T, Saito Y, et al. WNT pathway gene mutations are associated with the presence of dysplasia in colorectal sessile serrated adenoma/polyps. Am J Surg Pathol. 2017;41(9):1188-1197.
doi pubmed - Murakami T, Mitomi H, Saito T, Takahashi M, Sakamoto N, Fukui N, Yao T, et al. Distinct WNT/beta-catenin signaling activation in the serrated neoplasia pathway and the adenoma-carcinoma sequence of the colorectum. Mod Pathol. 2015;28(1):146-158.
doi pubmed - Whitehall VL, Walsh MD, Young J, Leggett BA, Jass JR. Methylation of O-6-methylguanine DNA methyltransferase characterizes a subset of colorectal cancer with low-level DNA microsatellite instability. Cancer Res. 2001;61(3):827-830.
- Hashimoto T, Ogawa R, Yoshida H, Taniguchi H, Kojima M, Saito Y, Sekine S. Acquisition of WNT pathway gene alterations coincides with the transition from precursor polyps to traditional serrated adenomas. Am J Surg Pathol. 2019;43(1):132-139.
doi pubmed - Torlakovic EE, Gomez JD, Driman DK, Parfitt JR, Wang C, Benerjee T, Snover DC. Sessile serrated adenoma (SSA) vs. traditional serrated adenoma (TSA). Am J Surg Pathol. 2008;32(1):21-29.
doi pubmed - The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58(6 Suppl):S3-43.
doi - van Doorn SC, Hazewinkel Y, East JE, van Leerdam ME, Rastogi A, Pellise M, Sanduleanu-Dascalescu S, et al. Polyp morphology: an interobserver evaluation for the Paris classification among international experts. Am J Gastroenterol. 2015;110(1):180-187.
doi pubmed - Aziz Aadam A, Wani S, Kahi C, Kaltenbach T, Oh Y, Edmundowicz S, Peng J, et al. Physician assessment and management of complex colon polyps: a multicenter video-based survey study. Am J Gastroenterol. 2014;109(9):1312-1324.
doi pubmed - Bartel MJ, Brahmbhatt BS, Wallace MB. Management of colorectal T1 carcinoma treated by endoscopic resection from the Western perspective. Dig Endosc. 2016;28(3):330-341.
doi pubmed - Matsuda T, Fukuzawa M, Uraoka T, Nishi M, Yamaguchi Y, Kobayashi N, Ikematsu H, et al. Risk of lymph node metastasis in patients with pedunculated type early invasive colorectal cancer: a retrospective multicenter study. Cancer Sci. 2011;102(9):1693-1697.
doi pubmed - Chandrasekhara V, Ginsberg GG. Endoscopic mucosal resection: not your father's polypectomy anymore. Gastroenterology. 2011;141(1):42-49.
doi pubmed - Asge Technology Committee, Hwang JH, Konda V, Abu Dayyeh BK, Chauhan SS, Enestvedt BK, Fujii-Lau LL, et al. Endoscopic mucosal resection. Gastrointest Endosc. 2015;82(2):215-226.
doi pubmed - Asge Technology Committee, Kantsevoy SV, Adler DG, Conway JD, Diehl DL, Farraye FA, Kwon R, et al. Endoscopic mucosal resection and endoscopic submucosal dissection. Gastrointest Endosc. 2008;68(1):11-18.
doi pubmed - Uraoka T, Parra-Blanco A, Yahagi N. Colorectal endoscopic submucosal dissection: is it suitable in western countries? J Gastroenterol Hepatol. 2013;28(3):406-414.
doi pubmed - Kim ER, Chang DK. Management of Complications of Colorectal Submucosal Dissection. Clin Endosc. 2019;52(2):114-119.
doi pubmed - Cooper HS. Surgical pathology of endoscopically removed malignant polyps of the colon and rectum. Am J Surg Pathol. 1983;7(7):613-623.
doi pubmed - Kashida H, Kudo SE. Early colorectal cancer: concept, diagnosis, and management. Int J Clin Oncol. 2006;11(1):1-8.
doi pubmed - Haggitt RC, Glotzbach RE, Soffer EE, Wruble LD. Prognostic factors in colorectal carcinomas arising in adenomas: implications for lesions removed by endoscopic polypectomy. Gastroenterology. 1985;89(2):328-336.
doi - Kyzer S, Begin LR, Gordon PH, Mitmaker B. The care of patients with colorectal polyps that contain invasive adenocarcinoma. Endoscopic polypectomy or colectomy? Cancer. 1992;70(8):2044-2050.
doi - Nivatvongs S, Rojanasakul A, Reiman HM, Dozois RR, Wolff BG, Pemberton JH, Beart RW, Jr., et al. The risk of lymph node metastasis in colorectal polyps with invasive adenocarcinoma. Dis Colon Rectum. 1991;34(4):323-328.
doi pubmed - Kudo S. Endoscopic mucosal resection of flat and depressed types of early colorectal cancer. Endoscopy. 1993;25(7):455-461.
doi pubmed - Kikuchi R, Takano M, Takagi K, Fujimoto N, Nozaki R, Fujiyoshi T, Uchida Y. Management of early invasive colorectal cancer. Risk of recurrence and clinical guidelines. Dis Colon Rectum. 1995;38(12):1286-1295.
doi pubmed - Nascimbeni R, Burgart LJ, Nivatvongs S, Larson DR. Risk of lymph node metastasis in T1 carcinoma of the colon and rectum. Dis Colon Rectum. 2002;45(2):200-206.
doi pubmed - Kitajima K, Fujimori T, Fujii S, Takeda J, Ohkura Y, Kawamata H, Kumamoto T, et al. Correlations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma: a Japanese collaborative study. J Gastroenterol. 2004;39(6):534-543.
doi pubmed - Beaton C, Twine CP, Williams GL, Radcliffe AG. Systematic review and meta-analysis of histopathological factors influencing the risk of lymph node metastasis in early colorectal cancer. Colorectal Dis. 2013;15(7):788-797.
doi pubmed - Bosch SL, Teerenstra S, de Wilt JH, Cunningham C, Nagtegaal ID. Predicting lymph node metastasis in pT1 colorectal cancer: a systematic review of risk factors providing rationale for therapy decisions. Endoscopy. 2013;45(10):827-834.
doi pubmed - Pai RK, Cheng YW, Jakubowski MA, Shadrach BL, Plesec TP, Pai RK. Colorectal carcinomas with submucosal invasion (pT1): analysis of histopathological and molecular factors predicting lymph node metastasis. Mod Pathol. 2017;30(1):113-122.
doi pubmed - Hashiguchi Y, Muro K, Saito Y, Ito Y, Ajioka Y, Hamaguchi T, Hasegawa K, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2020;25(1):1-42.
doi pubmed - Watanabe T, Itabashi M, Shimada Y, Tanaka S, Ito Y, Ajioka Y, Hamaguchi T, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol. 2015;20(2):207-239.
doi pubmed - Fogt F, Zimmerman RL, Ross HM, Daly T, Gausas RE. Identification of lymphatic vessels in malignant, adenomatous and normal colonic mucosa using the novel immunostain D2-40. Oncol Rep. 2004;11(1):47-50.
doi pubmed - Di Gregorio C, Bonetti LR, de Gaetani C, Pedroni M, Kaleci S, Ponz de Leon M. Clinical outcome of low- and high-risk malignant colorectal polyps: results of a population-based study and meta-analysis of the available literature. Intern Emerg Med. 2014;9(2):151-160.
doi pubmed - Cooper HS, Deppisch LM, Gourley WK, Kahn EI, Lev R, Manley PN, Pascal RR, et al. Endoscopically removed malignant colorectal polyps: clinicopathologic correlations. Gastroenterology. 1995;108(6):1657-1665.
doi - Butte JM, Tang P, Gonen M, Shia J, Schattner M, Nash GM, Temple LK, et al. Rate of residual disease after complete endoscopic resection of malignant colonic polyp. Dis Colon Rectum. 2012;55(2):122-127.
doi pubmed - Bujanda L, Cosme A, Gil I, Arenas-Mirave JI. Malignant colorectal polyps. World J Gastroenterol. 2010;16(25):3103-3111.
doi pubmed - Cunningham KN, Mills LR, Schuman BM, Mwakyusa DH. Long-term prognosis of well-differentiated adenocarcinoma in endoscopically removed colorectal adenomas. Dig Dis Sci. 1994;39(9):2034-2037.
doi pubmed - Netzer P, Forster C, Biral R, Ruchti C, Neuweiler J, Stauffer E, Schonegg R, et al. Risk factor assessment of endoscopically removed malignant colorectal polyps. Gut. 1998;43(5):669-674.
doi pubmed - Volk EE, Goldblum JR, Petras RE, Carey WD, Fazio VW. Management and outcome of patients with invasive carcinoma arising in colorectal polyps. Gastroenterology. 1995;109(6):1801-1807.
doi - Choi PW, Yu CS, Jang SJ, Jung SH, Kim HC, Kim JC. Risk factors for lymph node metastasis in submucosal invasive colorectal cancer. World J Surg. 2008;32(9):2089-2094.
doi pubmed - Ishikawa Y, Akishima-Fukasawa Y, Ito K, Akasaka Y, Yokoo T, Ishii T, Toho Study Group for Cancer Biological B. Histopathologic determinants of regional lymph node metastasis in early colorectal cancer. Cancer. 2008;112(4):924-933.
doi pubmed - Kim JH, Cheon JH, Kim TI, Baik SH, Kim NK, Kim H, Kim WH. Effectiveness of radical surgery after incomplete endoscopic mucosal resection for early colorectal cancers: a clinical study investigating risk factors of residual cancer. Dig Dis Sci. 2008;53(11):2941-2946.
doi pubmed - Yamamoto S, Watanabe M, Hasegawa H, Baba H, Yoshinare K, Shiraishi J, Kitajima M. The risk of lymph node metastasis in T1 colorectal carcinoma. Hepatogastroenterology. 2004;51(58):998-1000.
- Walgenbach-Bruenagel G, Tolba RH, Varnai AD, Bollmann M, Hirner A, Walgenbach KJ. Detection of lymphatic invasion in early stage primary colorectal cancer with the monoclonal antibody D2-40. Eur Surg Res. 2006;38(5):438-444.
doi pubmed - Kaiserling E, Krober S, Geleff S. Lymphatic vessels in the colonic mucosa in ulcerative colitis. Lymphology. 2003;36(2):52-61.
- Cooper HS, Deppisch LM, Kahn EI, Lev R, Manley PN, Pascal RR, Qizilbash AH, et al. Pathology of the malignant colorectal polyp. Hum Pathol. 1998;29(1):15-26.
doi - Muller S, Chesner IM, Egan MJ, Rowlands DC, Collard MJ, Swarbrick ET, Newman J. Significance of venous and lymphatic invasion in malignant polyps of the colon and rectum. Gut. 1989;30(10):1385-1391.
doi pubmed - Cooper HS. Pathologic issues in the treatment of endoscopically removed malignant colorectal polyps. J Natl Compr Canc Netw. 2007;5(9):991-996.
doi pubmed - Lugli A, Kirsch R, Ajioka Y, Bosman F, Cathomas G, Dawson H, El Zimaity H, et al. Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod Pathol. 2017;30(9):1299-1311.
doi pubmed - Ueno H, Mochizuki H, Hashiguchi Y, Shimazaki H, Aida S, Hase K, Matsukuma S, et al. Risk factors for an adverse outcome in early invasive colorectal carcinoma. Gastroenterology. 2004;127(2):385-394.
doi pubmed - Choi DH, Sohn DK, Chang HJ, Lim SB, Choi HS, Jeong SY. Indications for subsequent surgery after endoscopic resection of submucosally invasive colorectal carcinomas: a prospective cohort study. Dis Colon Rectum. 2009;52(3):438-445.
doi pubmed - Graham RP, Vierkant RA, Tillmans LS, Wang AH, Laird PW, Weisenberger DJ, Lynch CF, et al. Tumor budding in colorectal carcinoma: confirmation of prognostic significance and histologic cutoff in a population-based cohort. Am J Surg Pathol. 2015;39(10):1340-1346.
doi pubmed - Koelzer VH, Zlobec I, Lugli A. Tumor budding in colorectal cancer—ready for diagnostic practice? Hum Pathol. 2016;47(1):4-19.
doi pubmed - Petrelli F, Pezzica E, Cabiddu M, Coinu A, Borgonovo K, Ghilardi M, Lonati V, et al. Tumour budding and survival in Stage II colorectal cancer: a systematic review and pooled analysis. J Gastrointest Cancer. 2015;46(3):212-218.
doi pubmed - Ueno H, Mochizuki H, Hashiguchi Y, Ishiguro M, Kajiwara Y, Sato T, Shimazaki H, et al. Histological grading of colorectal cancer: a simple and objective method. Ann Surg. 2008;247(5):811-818.
doi pubmed - Ueno H, Hase K, Hashiguchi Y, Shimazaki H, Tanaka M, Miyake O, Masaki T, et al. Site-specific tumor grading system in colorectal cancer: multicenter pathologic review of the value of quantifying poorly differentiated clusters. Am J Surg Pathol. 2014;38(2):197-204.
doi pubmed - Ueno H, Kajiwara Y, Shimazaki H, Shinto E, Hashiguchi Y, Nakanishi K, Maekawa K, et al. New criteria for histologic grading of colorectal cancer. Am J Surg Pathol. 2012;36(2):193-201.
doi pubmed - Barresi V, Reggiani Bonetti L, Ieni A, Caruso RA, Tuccari G. Poorly Differentiated Clusters: Clinical Impact in Colorectal Cancer. Clin Colorectal Cancer. 2017;16(1):9-15.
doi pubmed - Haupt B, Ro JY, Schwartz MR, Shen SS. Colorectal adenocarcinoma with micropapillary pattern and its association with lymph node metastasis. Mod Pathol. 2007;20(7):729-733.
doi pubmed - Verdu M, Roman R, Calvo M, Rodon N, Garcia B, Gonzalez M, Vidal A, et al. Clinicopathological and molecular characterization of colorectal micropapillary carcinoma. Mod Pathol. 2011;24(5):729-738.
doi pubmed - Pyo JS, Park MJ, Kang DW. The clinicopathological significance of micropapillary pattern in colorectal cancers. Hum Pathol. 2018;77:159-165.
doi pubmed - Ueno H, Kanemitsu Y, Sekine S, Ishiguro M, Ito E, Hashiguchi Y, Kondo F, et al. Desmoplastic pattern at the tumor front defines poor-prognosis subtypes of colorectal cancer. Am J Surg Pathol. 2017;41(11):1506-1512.
doi pubmed - Nearchou IP, Kajiwara Y, Mochizuki S, Harrison DJ, Caie PD, Ueno H. Novel internationally verified method reports desmoplastic reaction as the most significant prognostic feature for disease-specific survival in Stage II colorectal cancer. Am J Surg Pathol. 2019;43(9):1239-1248.
doi pubmed - Ueno H, Shinto E, Shimazaki H, Kajiwara Y, Sueyama T, Yamamoto J, Hase K. Histologic categorization of desmoplastic reaction: its relevance to the colorectal cancer microenvironment and prognosis. Ann Surg Oncol. 2015;22(5):1504-1512.
doi pubmed - Fenoglio CM, Kaye GI, Lane N. Distribution of human colonic lymphatics in normal, hyperplastic, and adenomatous tissue. Its relationship to metastasis from small carcinomas in pedunculated adenomas, with two case reports. Gastroenterology. 1973;64(1):51-66.
doi - Lewin MR, Fenton H, Burkart AL, Sheridan T, Abu-Alfa AK, Montgomery EA. Poorly differentiated colorectal carcinoma with invasion restricted to lamina propria (intramucosal carcinoma): a follow-up study of 15 cases. Am J Surg Pathol. 2007;31(12):1882-1886.
doi pubmed - Laufman H, Saphir O. Primary linitis plastica type of carcinoma of the colon. AMA Arch Surg. 1951;62(1):79-91.
doi pubmed - Messerini L, Palomba A, Zampi G. Primary signet-ring cell carcinoma of the colon and rectum. Dis Colon Rectum. 1995;38(11):1189-1192.
doi pubmed - Nissan A, Guillem JG, Paty PB, Wong WD, Cohen AM. Signet-ring cell carcinoma of the colon and rectum: a matched control study. Dis Colon Rectum. 1999;42(9):1176-1180.
doi pubmed - Fu KI, Sano Y, Kato S, Saito H, Ochiai A, Fujimori T, Saito Y, et al. Primary signet-ring cell carcinoma of the colon at early stage: a case report and a review of the literature. World J Gastroenterol. 2006;12(21):3446-3449.
doi pubmed - Kim JH, Park SJ, Park MI, Moon W, Kim SE. Early-stage primary signet ring cell carcinoma of the colon. World J Gastroenterol. 2013;19(24):3895-3898.
doi pubmed - Mai KT, Isotalo PA, Guindi M, Burns BF, Parks W. Intestinal epithelial lesions associated with signet ring cell carcinoma of the colon and small intestine. Pathology. 2002;34(1):51-56.
doi pubmed - Nakamura T, Nakano G, Sakamoto K. Adenoma of the rectum with multiple foci of signet-ring cell carcinoma. Report of a case. Dis Colon Rectum. 1983;26(8):529-532.
doi pubmed - Shimaoka S, Niihara T, Tashiro K, Matsuda A, Nioh T, Ohi H, Nishimata Y, et al. Signet-ring cell carcinoma of the colon 7mm in size with peritonitis carcinomatosa. J Gastroenterol. 2002;37(7):550-555.
doi pubmed - Shimoda T, Ikegami M, Fujisaki J, Matsui T, Aizawa S, Ishikawa E. Early colorectal carcinoma with special reference to its development de novo. Cancer. 1989;64(5):1138-1146.
doi - Tandon M, Sostek M, Klein MA. Focus of signet ring cell carcinoma in an adenoma of the sigmoid colon. Arch Pathol Lab Med. 1999;123(10):957-959.
- Tsujinaka Y, Tsuchiya S, Ooki S, Oomi Y, Kaneko H, Eguchi H, Kikkou T. IIc type of early carcinoma of the rectum originating"de novo", report of a case. Stomach and Intestine. 1983;18:211-217.
- Jass JR. Do all colorectal carcinomas arise in preexisting adenomas? World J Surg. 1989;13(1):45-51.
doi pubmed - Zou Y, Shi H, Costedio M, Liu X. An intramucosal large cell neuroendocrine carcinoma arising within a tubulovillous adenoma with high-grade dysplasia of the rectosigmoid colon treated with polypectomy. Journal of Medical Cases. 2016;7(5):174-177.
doi - Gaffey MJ, Mills SE, Lack EE. Neuroendocrine carcinoma of the colon and rectum. A clinicopathologic, ultrastructural, and immunohistochemical study of 24 cases. Am J Surg Pathol. 1990;14(11):1010-1023.
doi pubmed - Rubio CA. Ectopic colonic mucosa in ulcerative colitis and in Crohn's disease of the colon. Dis Colon Rectum. 1984;27(3):182-186.
doi pubmed - De Petris G, Lev R, Quirk DM, Ferbend PR, Butmarc JR, Elenitoba-Johnson K. Lymphoepithelioma-like carcinoma of the colon in a patient with hereditary nonpolyposis colorectal cancer. Arch Pathol Lab Med. 1999;123(8):720-724.
- McCarthy AJ, Chetty R. Gut-associated lymphoid tissue or so-called "dome" carcinoma of the colon: Review. World J Gastrointest Oncol. 2019;11(1):59-70.
doi pubmed - Lee HE, Wu TT, Chandan VS, Torbenson MS, Mounajjed T. Colonic adenomatous polyps involving submucosal lymphoglandular complexes: a diagnostic pitfall. Am J Surg Pathol. 2018;42(8):1083-1089.
doi pubmed - Pulitzer M, Xu R, Suriawinata AA, Waye JD, Harpaz N. Microcarcinoids in large intestinal adenomas. Am J Surg Pathol. 2006;30(12):1531-1536.
doi pubmed - Kim MJ, Lee EJ, Kim DS, Lee DH, Youk EG, Kim HJ. Composite intestinal adenoma-microcarcinoid in the colon and rectum: a case series and historical review. Diagn Pathol. 2017;12(1):78.
doi pubmed - Lin J, Goldblum JR, Bennett AE, Bronner MP, Liu X. Composite intestinal adenoma-microcarcinoid. Am J Surg Pathol. 2012;36(2):292-295.
doi pubmed - Lyda MH, Fenoglio-Preiser CM. Adenoma-carcinoid tumors of the colon. Arch Pathol Lab Med. 1998;122(3):262-265.
- Salaria SN, Abu Alfa AK, Alsaigh NY, Montgomery E, Arnold CA. Composite intestinal adenoma-microcarcinoid clues to diagnosing an under-recognised mimic of invasive adenocarcinoma. J Clin Pathol. 2013;66(4):302-306.
doi pubmed - Thosani N, Rao B, Ertan A, Guha S. Wide spectrum of neuroendocrine differentiation in identical appearing colon polyps: A report of 2 mixed endocrine-glandular polyps. Turk J Gastroenterol. 2014;25(Suppl 1):242-243.
doi pubmed - Fu Z, Saade R, Koo BH, Jennings TA, Lee H. Incidence of composite intestinal adenoma-microcarcinoid in 158 surgically resected polyps and its association with squamous morule. Ann Diagn Pathol. 2019;42:69-74.
doi pubmed - Knoernschild HE. The use of a tattooing instrument for marking colonic mucosa. Am J Surg. 1962;103:83-85.
doi - Ponsky JL, King JF. Endoscopic marking of colonic lesions. Gastrointest Endosc. 1975;22(1):42-43.
doi - Sauntry JP, Knudtson KP. A technique for marking the mucosa of the gastrointestinal tract after polypectomy. Cancer. 1958;11(3):607-610.
doi - Coman E, Brandt LJ, Brenner S, Frank M, Sablay B, Bennett B. Fat necrosis and inflammatory pseudotumor due to endoscopic tattooing of the colon with india ink. Gastrointest Endosc. 1991;37(1):65-68.
doi - Gopal DV, Morava-Protzner I, Miller HA, Hemphill DJ. Idiopathic inflammatory bowel disease associated with colonic tattooing with india ink preparation—case report and review of literature. Gastrointest Endosc. 1999;49(5):636-639.
doi - Tutticci N, Cameron D, Croese J, Roche E. Peritoneal deposits with carbon pigmentation associated with endoscopic submucosal tattooing of a rectal cancer. Endoscopy. 2010;42(Suppl 2):E136.
doi pubmed - Askin MP, Waye JD, Fiedler L, Harpaz N. Tattoo of colonic neoplasms in 113 patients with a new sterile carbon compound. Gastrointest Endosc. 2002;56(3):339-342.
doi - Sun B. Endoscopic tattooing: a risk for tumor implantation. Int J Colorectal Dis. 2020;35(3):571-574.
doi pubmed - Fennerty MB, Sampliner RE, Hixson LJ, Garewal HS. Effectiveness of India ink as a long-term colonic mucosal marker. Am J Gastroenterol. 1992;87(1):79-81.
- Trakarnsanga A, Akaraviputh T. Endoscopic tattooing of colorectal lesions: Is it a risk-free procedure? World J Gastrointest Endosc. 2011;3(12):256-260.
doi pubmed - Castrodad-Rodriguez CA, Panarelli NC, Gersten AJ, Liu Q, Feely M, Jabbour TE. Features of endoscopic procedure site reaction associated with a recently approved submucosal lifting agent. Mod Pathol. 2020;33(8):1581-1588.
doi pubmed - Pezhouh MK, Burgart LJ, Chiu K, Cohen DA, Hutchings DA, Sanderson SO, Shirazi M, et al. Characterization of novel injectable lifting agents used in colonic polyp removal: an emerging amyloid mimic. Am J Surg Pathol. 2020;44(6):793-798.
doi pubmed - Cypher L, Sun S, Forster E, Hoffman B, Lewin D. Submucosal lifting agent Orise gel remnants histopathologically mimic mucin and malignancy: a case series. Am J Clin Pathol. 2019;152(suppl 1):S73.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


