| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website http://www.gastrores.org |
Original Article
Volume 11, Number 2, April 2018, pages 106-111
Suspected Blood Indicator to Identify Active Gastrointestinal Bleeding: A Prospective Validation
Samuel Hana, b, Julien Faheda, David R Cavea
aDivision of Gastroenterology, University of Massachusetts Medical Center, Worcester, MA, USA
bCorresponding Author: Samuel Han, Division of Gastroenterology and Hepatology, University of Colorado Anschutz Medical Center, Mail Stop F735, 1635 Aurora Court, Room 2.031, Aurora, CO 80045, USA
Manuscript submitted December 15, 2017, accepted December 20, 2017
Short title: SBI for Active GI Bleeding
doi: https://doi.org/10.14740/gr949w
| Abstract | ▴Top |
Background: The suspected blood indicator (SBI) function in the RAPID Reader v8.3 program was designed to quickly identify the presence of blood in video capsule endoscopy. While previous retrospective studies have shown that the SBI function was accurate in detecting the presence of active bleeding in the small bowel, its specificity and sensitivity were poor.
Methods: An initial retrospective review (phase 1) compared 115 patients with active gastrointestinal bleeding seen on video capsule endoscopy (VCE) to 115 patients with no active bleeding seen on VCE to produce a highly accurate algorithm. A prospective study (phase 2) was then performed by applying the algorithm to 100 consecutive patients who received VCE for the following indications: obscure bleeding, iron deficiency anemia, melena, and hematochezia.
Results: The initial retrospective review found that eight contiguous SBI markers had a specificity of 100% in identifying active gastrointestinal bleeding regardless of the total number of SBI markers, while two or more contiguous SBI markers had a sensitivity of 96.5%. Using a cutoff of eight contiguous SBI markers, the prospective arm found that there was a 100% sensitivity and specificity in detecting active gastrointestinal bleeding (P < 0.001).
Conclusions: The SBI function can greatly facilitate the identification of active gastrointestinal bleeding on VCE by using eight contiguous SBI markers as a cutoff for active bleeding.
Keywords: Video capsule endoscopy; Gastrointestinal bleeding; Small bowel endoscopy; Obscure bleeding; Small bowel bleeding
| Introduction | ▴Top |
Video capsule endoscopy (VCE) has become a primary tool in evaluating obscure gastrointestinal (GI) bleeding. The overall yield of VCE in identifying causes of obscure GI bleeding has been reported to range from 30-92% and is significantly higher than detection via push enteroscopy or other modalities [1, 2]. Lesions detected by VCE typically include small bowel angioectasias, ulcers, inflammation and tumors and may detect abnormalities in other organs such as esophagitis/gastritis [3]. As might be expected, the diagnostic yield is optimized if the capsule is deployed as close as possible to a bleeding episode [2-4].
A major drawback in VCE resides in the time-consuming and meticulous process of analyzing VCE videos. Typically involving over 50,000 images for the entire video, VCE requires not only a significant time commitment, but also an experienced reader adept at identifying abnormalities. The Given Imaging Rapid Reader program (RAPID Reader v.8.3, Given Imaging, Yoqneam, Israel, 2015) includes a software function known as “suspected blood indicator” (SBI) which is programmed to detect red-colored clusters of pixels in images and correspondingly sets markers, which appear as red bars, thus notifying the reader of what appears to be blood. A previous version of SBI had a low sensitivity in detecting active bleeding, and was upgraded in the current RAPID Reader program [5].
An accurate SBI function could greatly expedite VCE reading in detecting GI bleeding. This study evaluates the accuracy of SBI in detecting bleeding throughout the entire GI tract, while attempting to determine the number of contiguous SBI bars needed to accurately and consistently identify luminal blood related to active GI bleeding.
| Materials and Methods | ▴Top |
Phase 1 consisted of a single center retrospective review of consecutive patients who were found to have active bleeding on VCE reports during a period from January, 2015 to June, 2015. Indications for the VCE included obscure GI bleeding, iron deficiency anemia, melena, and hematochezia. A control group consisting of the same number of individuals from the same time period was also analyzed. This control group consisted of patients who received VCE for indications such as abdominal pain or concern for inflammatory bowel disease or celiac disease and were found to not have any active GI bleeding. The Institutional Review Board approved the study.
All patients received VCE under standard procedure as detailed by Iddan et al [6]. Preparation was limited to nil per os for 12 h. Digital videos of the VCE imaging had been read and analyzed using the RAPID Reader v8.3 software by one of two experienced clinicians who have reviewed over 2,000 VCE cases each. Active bleeding was defined as the finding of blood by one of the experienced clinicians as noted on the final report, which included the location of bleeding and if possible, the etiology of the bleeding. No active bleeding was defined as the absence of blood, which was also noted on the final report by one of two experienced clinicians. Two separate investigators (SH and JF) then analyzed the videos utilizing the SBI function, identifying SBI markers and their correlation with images displaying active bleeding (Fig. 1). SBI markers were then recorded to include true positive SBI markers as well as false positive SBI markers. False positive SBI markers were defined as red bars that were triggered by the SBI function, but were found on review to not have any blood in the frame. Using this data, a cutoff number for SBI markers with high sensitivity and specificity in identifying active GI bleeding was then calculated.
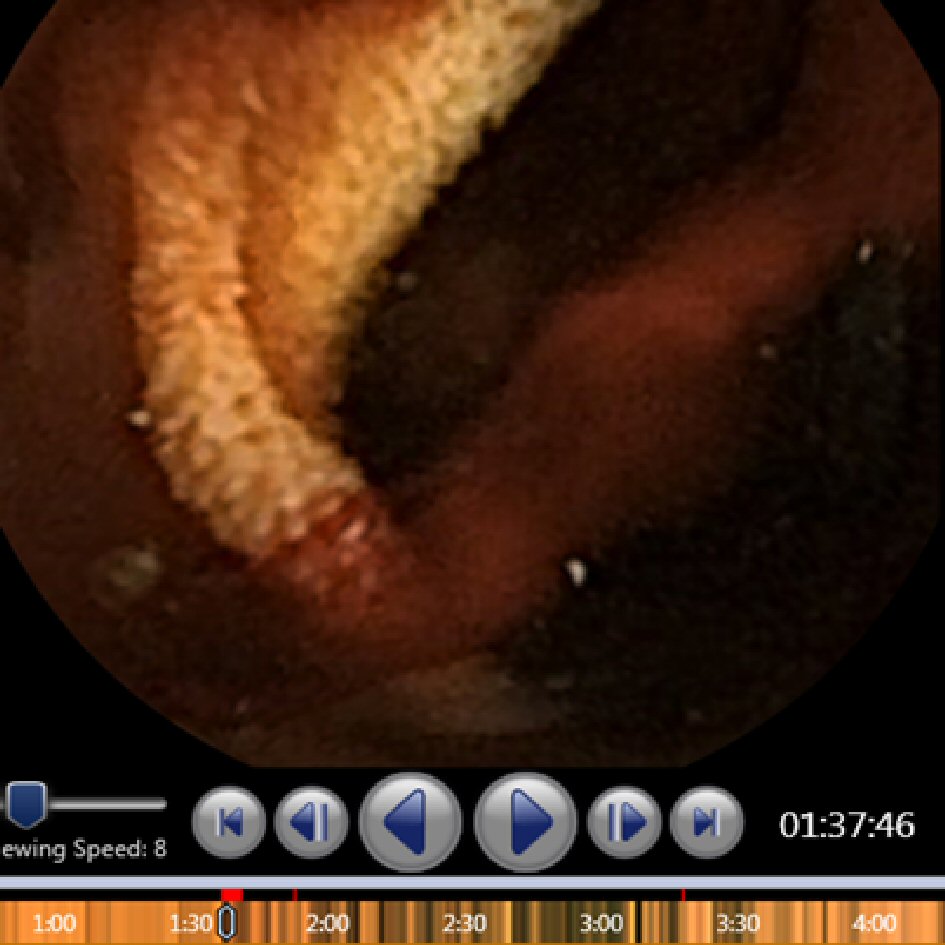 Click for large image | Figure 1. Example of active bleeding with suspected blood indicator markers (red bars). |
Upon completion of this retrospective review, the cutoff for SBI markers derived from phase 1 was then applied in a prospective validation (phase 2). Consecutive patients were enrolled from September, 2015 to December, 2015 under inclusion criteria consisting of the following indications: VCE for obscure GI bleeding, iron deficiency anemia, melena, or hematochezia. The VCE images were reviewed by the same two experienced investigators as described above, and SBI markers were then reviewed by the same two separate investigators as described above. SBI markers were again recorded to include true positive SBI markers as well as false positive SBI markers.
Statistical analysis
Student’s t-test and Fisher’s exact test were used to compare continuous and categorical variables, respectively. Sensitivity, specificity, positive predictive value and negative predictive value were calculated utilizing contingency tables. A P value less than 0.05 was considered to be statistically significant. All analysis was performed using STATA (StataCorp, College Station, Texas).
| Results | ▴Top |
Phase 1
The initial retrospective review (phase 1) consisted of video capsule data from 230 patients at our institution, a tertiary academic medical center. One half (n = 115) had active GI bleeding, while the other half was used as a control group. The SBI function was able to detect 100% of the 115 active GI bleeding cases documented on VCE. The most common finding on VCE in the GI bleeding group was angioectasia, which was found in 45 patients (39.1%) in the bleeding group compared to seven patients (6.09%) in the non-bleeding group. Other VCE findings are seen in Figure 2. VCE alone was able to detect the cause of bleeding in 53.7% in the active GI bleeding group, and the use of further modalities produced an overall determination rate of 79.1% for etiology of bleeding. Comparing the active bleeding group and the control group, the active bleeding group was significantly older (68.1 vs. 57 years, P < 0.001) and had significantly more contiguous SBI bars than the control group (21.1 vs. 1.2, P < 0.001) (Table 1). Patients in the active bleeding group also had a higher mean number of SBI markers than the control group, with a mean number of 21.2 SBI markers in the GI bleeding group and 1.2 SBI markers in the control group (P < 0.01). The distribution of contiguous SBI bars was also found to be significantly different between the two groups (P < 0.001) as seen in Figure 3.
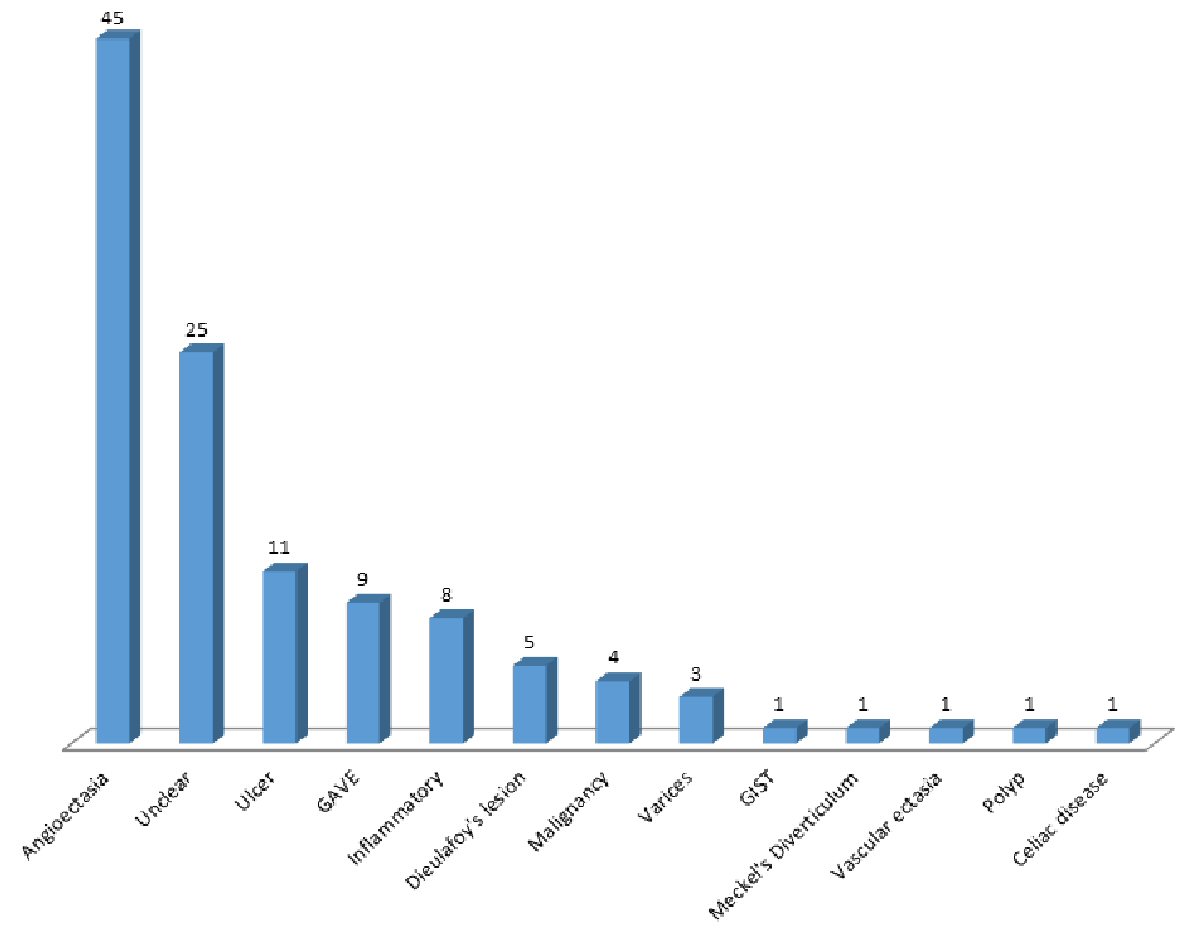 Click for large image | Figure 2. Etiology of gastrointestinal bleeding in phase 1. |
 Click to view | Table 1. Retrospective Comparison of Active Bleeding and Non-Bleeding Groups |
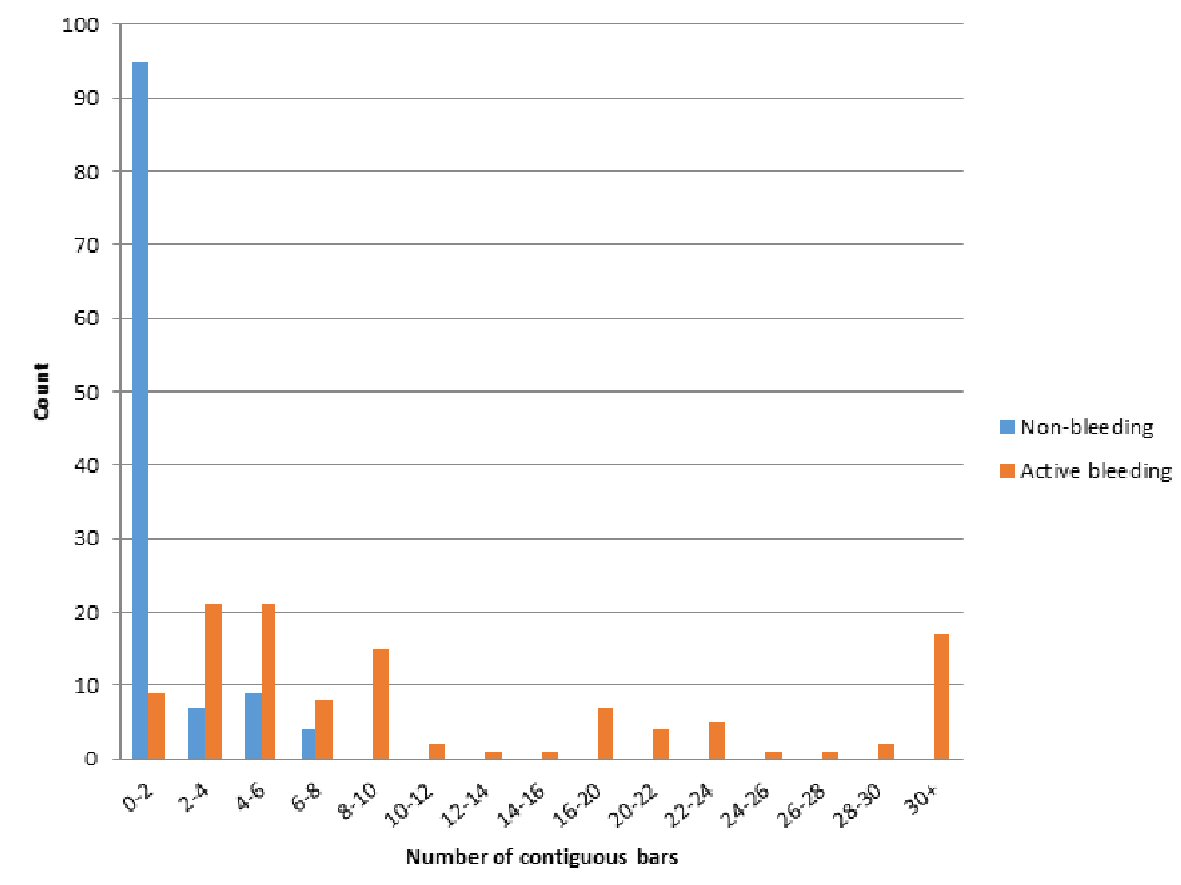 Click for large image | Figure 3. Distribution of contiguous bars. |
A ROC curve (Fig. 4) and contingency table (Table 2) determined that eight contiguous SBI bars or more had 100% specificity in identifying a GI bleed with 45.2% of the active GI bleeding cases having less than eight contiguous bars. Using eight continuous bars, sensitivity was 53%, positive predictive value was 100% and negative predictive value was 68.1%. On the other hand, it was determined that two contiguous SBI bars or more had 96.5% sensitivity and 66.1% specificity in identifying a GI bleed.
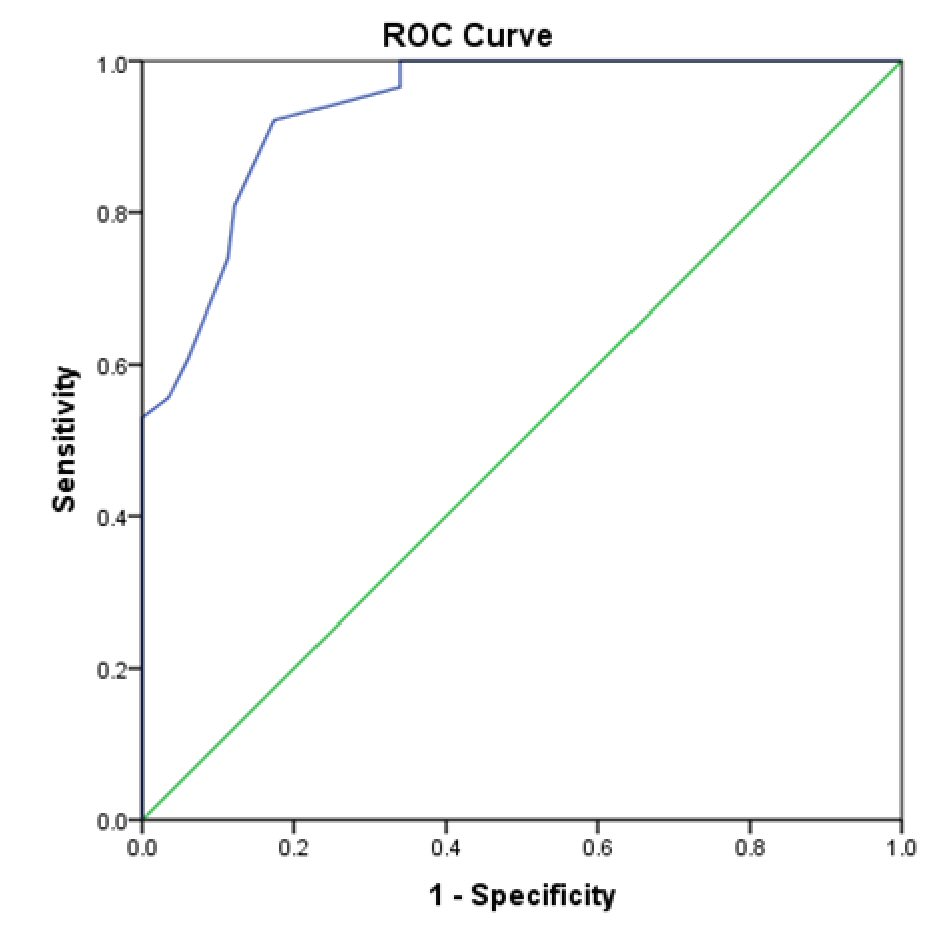 Click for large image | Figure 4. ROC curve of SBI accuracy in phase 1 in detecting active gastrointestinal bleeding. |
 Click to view | Table 2. Contingency Table Demonstrating Use of Eight Contiguous SBI Markers as Cutoff for Active Gastrointestinal Bleeding |
Phase 2
Phase 2 found a 100% sensitivity and specificity in identifying active GI bleeding using the formula of eight contiguous SBI markers derived from phase 1. This prospective arm detected 18 cases (18%) of active GI bleeding. In cases of active GI bleeding, the mean number of total bars, false positive bars, and contiguous bars was 37, 4.5 and 32.5, respectively (Table 3). The active bleeding group had a higher number of total SBI bars (37 vs. 4.9, P < 0.001) and contiguous bars (32.5 vs. 0.6, P < 0.001) than that of the non-bleeding group. The most common cause of bleeding found was angioectasia (38.9%) and other causes are displayed in Table 4.
 Click to view | Table 3. Comparison of Active Bleeding and Non-Bleeding Groups in Prospective Arm |
 Click to view | Table 4. Causes of Active Bleeding in Prospective Study |
| Discussion | ▴Top |
This study evaluated the capability of the SBI function in the RAPID Reader v8.3 software to accurately predict active GI bleeding. The SBI function was successfully able to detect all active GI bleeds and it appears that a series of eight contiguous SBI bars allows for 100% sensitivity and specificity.
Previously, Stein et al utilized the Quickview function in the RAPID Reader software v6.0 in conjunction with the SBI function and found an overall sensitivity of 100% in detecting 28 cases of active small bowel bleeding [7]. Tal et al utilized the SBI function alone and also found 100% sensitivity in detecting 42 cases of active small bowel bleeding, with a mean of 46.6 SBI bars in major bleeding cases and a mean of 36 SBI bars in minor bleeding cases [8]. Using that data, they created an optimal cutoff value of SBI number < 51 to correspond to a “no bleeding” group with > 51 SBI bars corresponding to a “true bleeding” group. This enabled a sensitivity of 79.1% and a specificity of 90.4%. Our study expands on these studies by evaluating bleeding throughout the entire GI tract and taking into account the importance of contiguous SBI bars. As blood flows through the GI tract from a particular source, the blood would be expected to appear in a consecutive series of images, reflecting the progression of the video capsule which also travels through the GI tract. This reflects the importance of the contiguous number of SBI bars in detecting an active GI bleed and implies that there could be a number of contiguous bars which would correspond well to active bleeding. In this study, we identified eight contiguous bars as representing the ideal number to effectively rule in an active GI bleed, and we found a contiguous series much easier to identify and count, as opposed to counting over 50 individual bars as suggested by Tal et al [8].
In terms of the detection capabilities of VCE, Liao et al performed a large meta-analysis including 22,840 VCE procedures and found a bleeding detection rate of 61% [9]. Lepileur et al looked at VCE procedures done for the indication of obscure GI bleeding and found that VCE was able to identify a cause of bleeding in 56% of the cases [3]. Our results were in line with these studies, as we were able to identify a source of bleeding in 53.7% of the retrospective cases and 68.8% in the prospective cases. The failure to detect bleeding more frequently reflects the timing of VCE and the intermittent nature of GI bleeding. With the use of further modalities such as double-balloon enteroscopy and spiral enteroscopy, the etiology was able to be elucidated in nearly 80% of both the retrospective and prospective cases in this study, which reflects the elusive nature of obscure GI bleeding.
The importance of this study stems from the possibility that VCE may be useful in the evaluation and triage of suspected GI bleeding, particularly in the emergency room (ER). Sung et al performed a randomized controlled trial evaluating the utility of VCE in patients presenting to the ER with suspected upper GI bleeding [10]. Patients receiving VCE were triaged based on real-time capsule results, with patients being discharged from the ER if there were no significant endoscopic findings. This feasibility study found that there was no difference in recurrent bleeding and 30-day mortality between patients who received VCE and those who received standard care, while hospitalization rates were significantly lower in the VCE group. While further trials need to be conducted to validate the use of VCE in acute GI bleeding, their study highlighted the benefit of using VCE early in the workup of GI bleeding. The SBI function could then prove very useful in the quick and accurate detection of acute bleeding, and help direct appropriate therapy, potentially improving patient outcomes, decreasing length of stay, and thereby reduce costs.
The presence of false positive bars presents the largest limitation of the SBI function. Slightly less than half of the active GI bleeding cases had false positive bars, and about 25% of the control group had false positive bars as well. In examining the false positive bars, the majority appear to be due to bubbles in the GI lumen, which appear to reflect light that is identified by the software as being red in color. However, not all bubbles elicit a response in the SBI bar; it appears that only a specific orientation of the camera in relation to the bubble will reflect the red-colored light. Nevertheless, only rarely did these bubbles produce a continuous series of SBI bars, which can be accommodated for by utilizing a cutoff of eight contiguous bars. Conversely, false negatives may indicate the failure of the SBI algorithm to detect colors that are slightly off-red. As blood can vary in color, the SBI is likely unable to detect all shades of red. Lastly, selection bias may have played a role in the increased sensitivity in phase 2 as there were only 18 cases of active bleeding in the prospective arm. Given that significant bleeding will span multiple frames and increase the likelihood of contiguous SBI markers, this likely reflects an inherent selection of patients in phase 2 with substantial bleeding.
Conclusions
In summary, the SBI function of the RAPID Reader v8.3 program provides a quick, pragmatic approach to identifying active GI bleeds. The quick identification of a contiguous series of eight red bars or more ensures detection of an active GI bleed, and the lack of a contiguous series of bars will help rule out GI bleeds. The presence of false positive bars, however, reinforces the importance of always reviewing the video capsule images, as bubbles are easy to identify. Since active GI bleeding always correlated with SBI markers, an appropriate capsule reading strategy should include early review of the SBI markers.
Conflict of Interest
None.
Financial Support
None.
Disclosure
Results of this study were presented in part at Digestive Disease Week 2015 and 2016.
| References | ▴Top |
- Triester SL, Leighton JA, Leontiadis GI, Fleischer DE, Hara AK, Heigh RI, Shiff AD, et al. A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with obscure gastrointestinal bleeding. Am J Gastroenterol. 2005;100(11):2407-2418.
doi pubmed - Pennazio M, Santucci R, Rondonotti E, Abbiati C, Beccari G, Rossini FP, De Franchis R. Outcome of patients with obscure gastrointestinal bleeding after capsule endoscopy: report of 100 consecutive cases. Gastroenterology. 2004;126(3):643-653.
doi pubmed - Lepileur L, Dray X, Antonietti M, Iwanicki-Caron I, Grigioni S, Chaput U, Di-Fiore A, et al. Factors associated with diagnosis of obscure gastrointestinal bleeding by video capsule enteroscopy. Clin Gastroenterol Hepatol. 2012;10(12):1376-1380.
doi pubmed - Singh A, Marshall C, Chaudhuri B, Okoli C, Foley A, Person SD, Bhattacharya K, et al. Timing of video capsule endoscopy relative to overt obscure GI bleeding: implications from a retrospective study. Gastrointest Endosc. 2013;77(5):761-766.
doi pubmed - Buscaglia JM, Giday SA, Kantsevoy SV, Clarke JO, Magno P, Yong E, Mullin GE. Performance characteristics of the suspected blood indicator feature in capsule endoscopy according to indication for study. Clin Gastroenterol Hepatol. 2008;6(3):298-301.
doi pubmed - Iddan G, Meron G, Glukhovsky A, Swain P. Wireless capsule endoscopy. Nature. 2000;405(6785):417.
doi pubmed - Stein AC, Appannagari A, Habib I, Semrad CE, Rubin DT. A rapid and accurate method to detect active small bowel gastrointestinal bleeding on video capsule endoscopy. Dig Dis Sci. 2014;59(10):2503-2507.
doi pubmed - Tal AO, Filmann N, Makhlin K, Hausmann J, Friedrich-Rust M, Herrmann E, Zeuzem S, et al. The capsule endoscopy "suspected blood indicator" (SBI) for detection of active small bowel bleeding: no active bleeding in case of negative SBI. Scand J Gastroenterol. 2014;49(9):1131-1135.
doi pubmed - Liao Z, Gao R, Xu C, Li ZS. Indications and detection, completion, and retention rates of small-bowel capsule endoscopy: a systematic review. Gastrointest Endosc. 2010;71(2):280-286.
doi pubmed - Sung JJ, Tang RS, Ching JY, Rainer TH, Lau JY. Use of capsule endoscopy in the emergency department as a triage of patients with GI bleeding. Gastrointest Endosc. 2016;84(6):907-913.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


