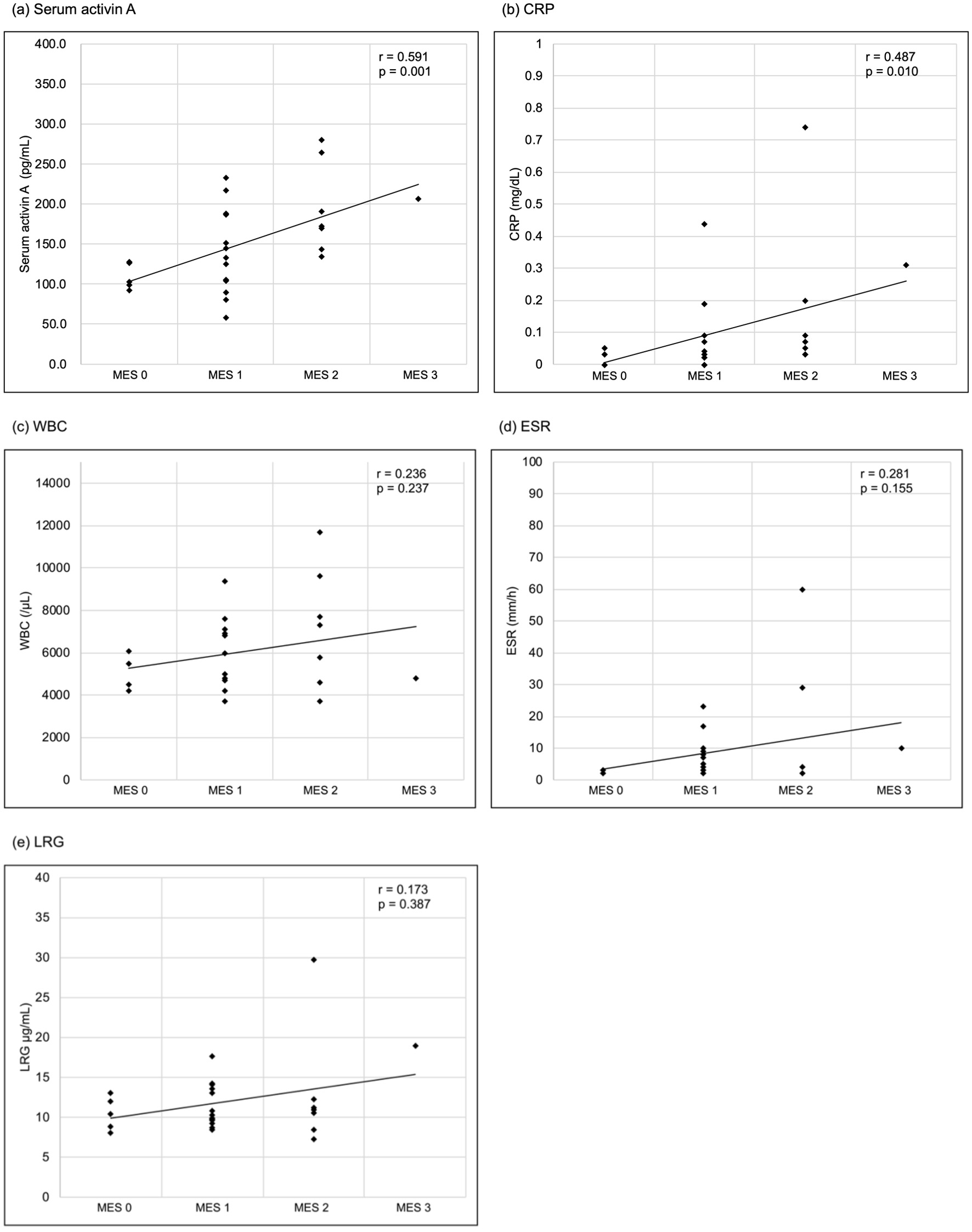
Figure 1. Correlations between laboratory parameters and the MES. (a) Serum activin A, (b) CRP, (c) WBC, (d) ESR, (e) LRG. Serum activin A and CRP significantly correlated with the MES (Spearman’s rank correlation coefficient r = 0.591, P = 0.001; r = 0.487, P = 0.010, respectively), while WBC, ESR and LRG had no significant correlation (r = 0.236, P = 0.237; r = 0.281, P = 0.155; r = 0.173, P = 0.387, respectively). CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; LRG: leucine-rich alpha-2 glycoprotein; MES: Mayo endoscopic score; WBC: white blood cell.
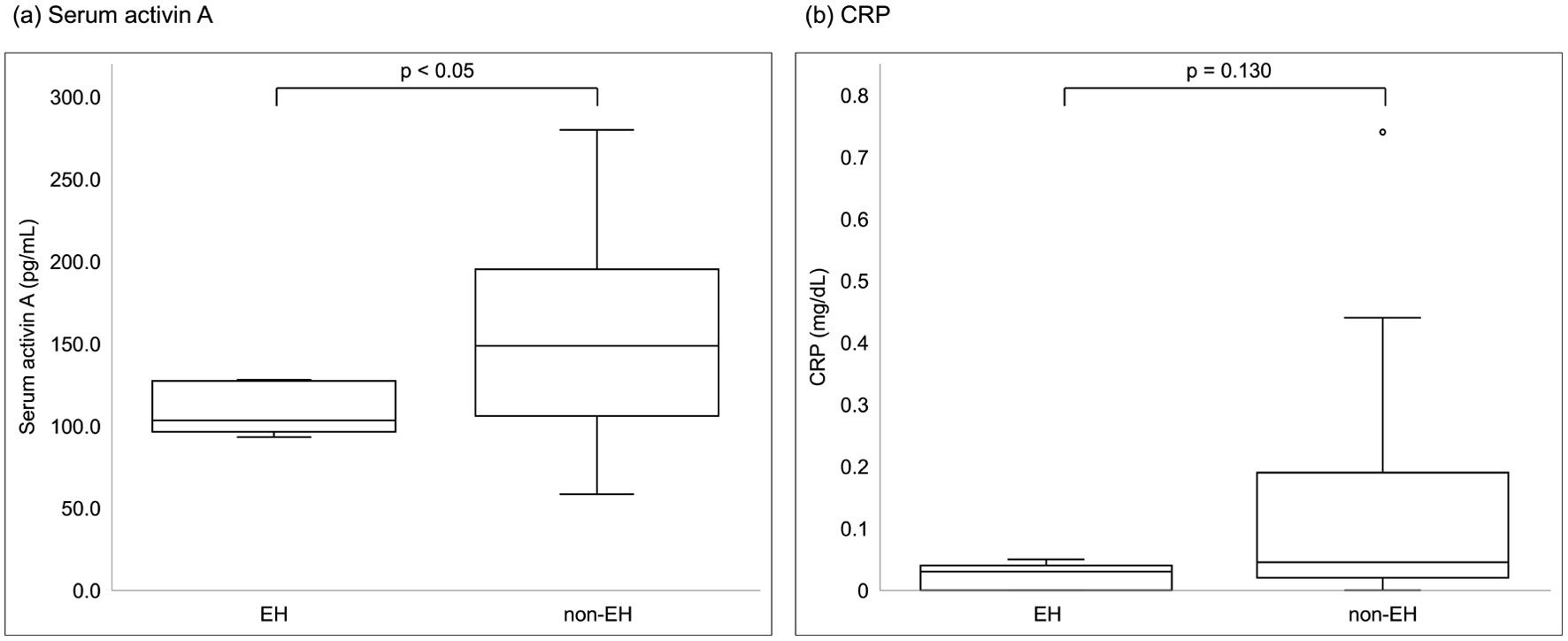
Figure 2. Comparisons of laboratory parameters between patients with EH and non-EH. (a) Serum activin A, (b) CRP. Among laboratory parameters, only serum activin A was significantly different between the groups (Mann-Whitney U test, P = 0.047). CRP: C-reactive protein; EH: endoscopic healing.
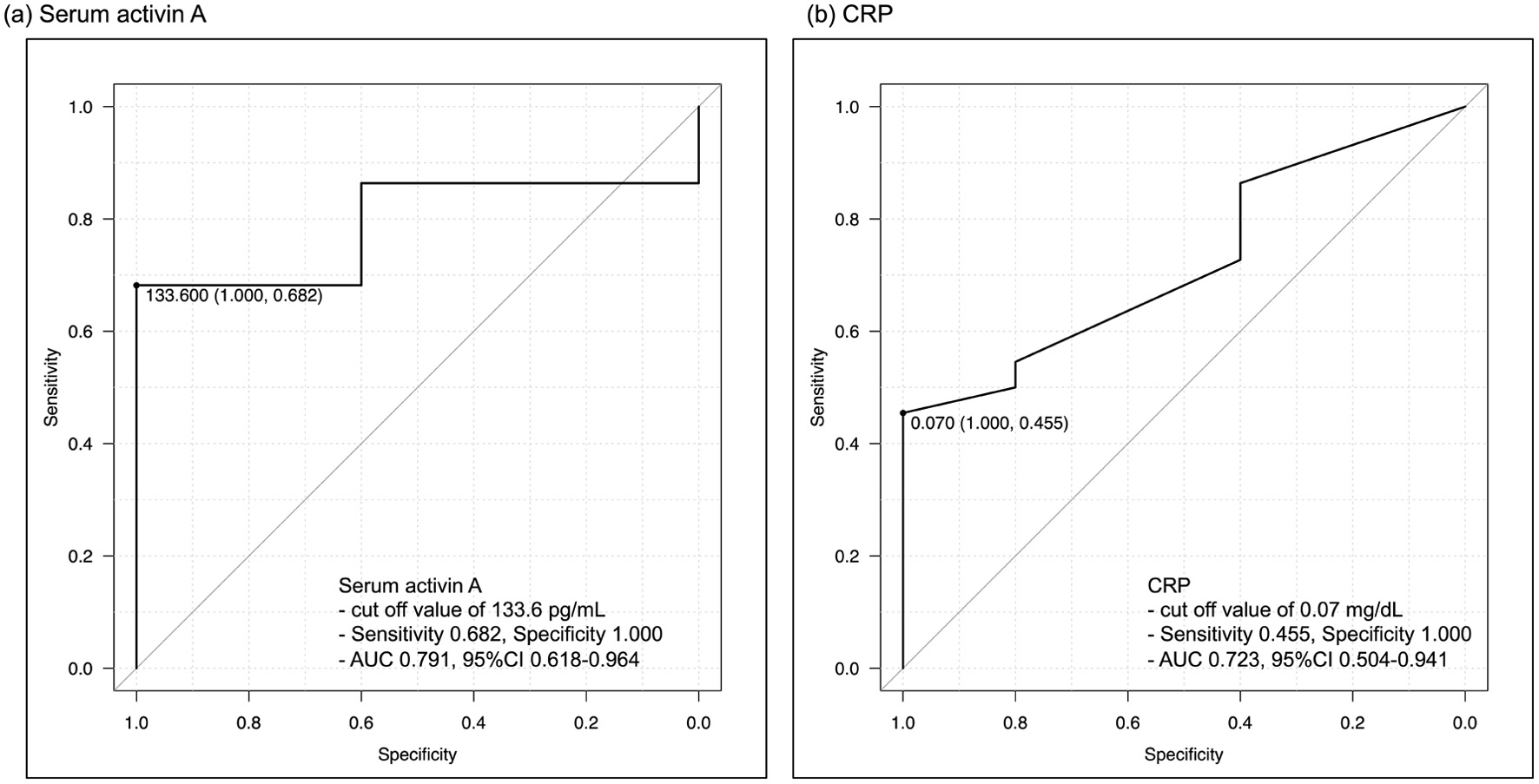
Figure 3. Receiver-operating characteristic curves for detecting non-EH. (a) Serum activin A, (b) CRP. The best cut-off value of serum activin A is 133.6 pg/mL, with a sensitivity of 0.682 and specificity of 1.000. The AUC of serum activin A is 0.791, while that of CRP is 0.723. AUC: area under the curve; CRP: C-reactive protein; EH: endoscopic healing.


