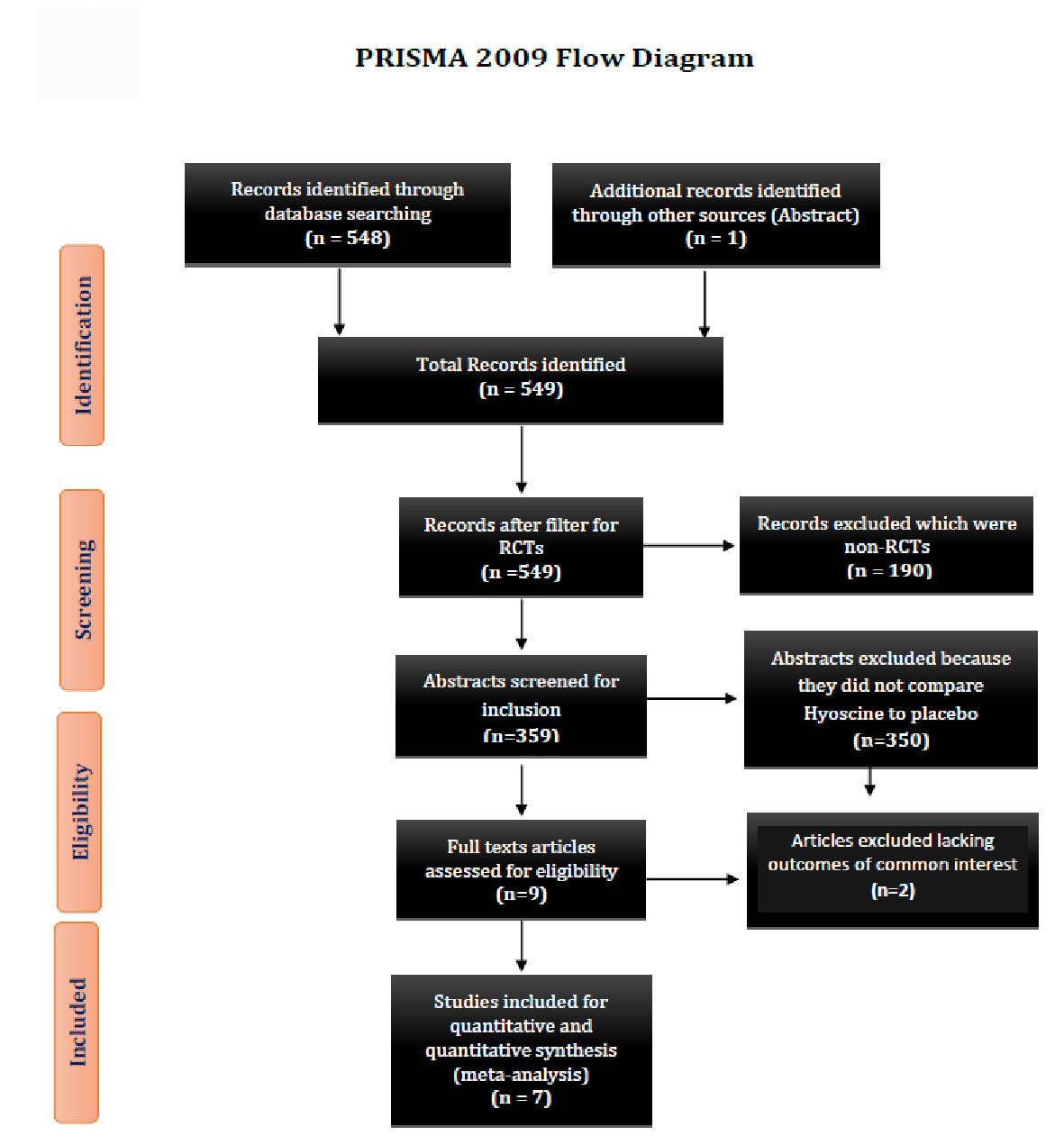
Figure 1. Showing the PRISMA 2009 study flow diagram. PRISMA: preferred reporting items for systematic reviews and meta-analyses; RCT: randomized control trial.
| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website http://www.gastrores.org |
Original Article
Volume 11, Number 4, August 2018, pages 295-304
Impact of Hyoscine Bromide Use on Polyp Detection Rate During Colonoscopy: A Systematic Review and Meta-Analysis
Figures

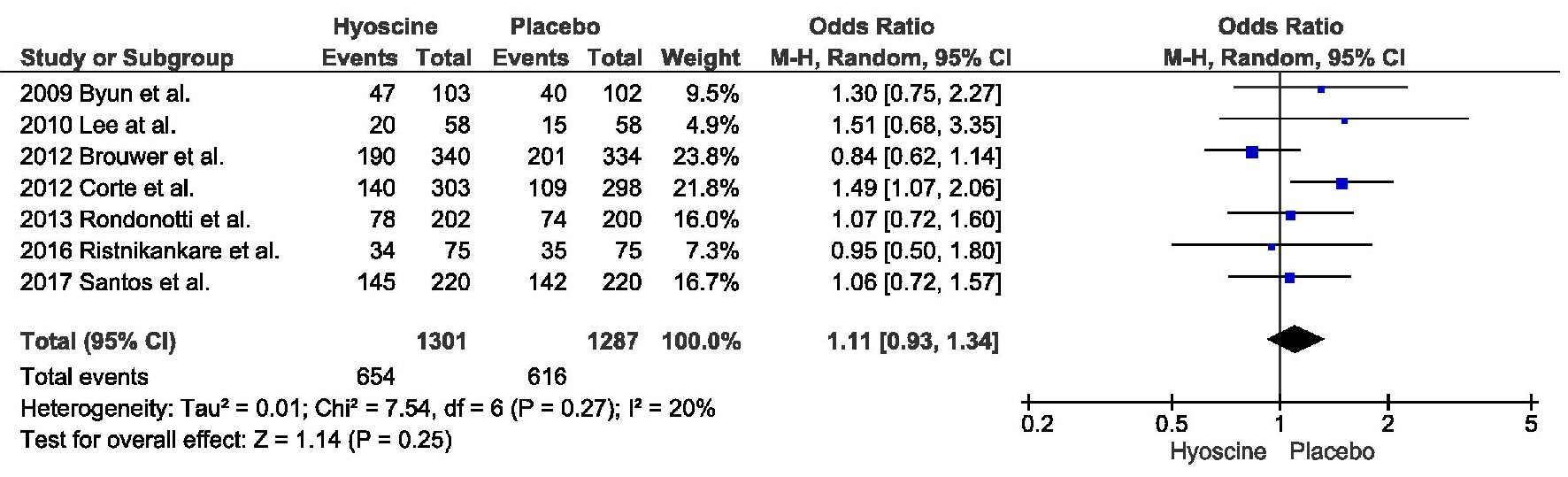
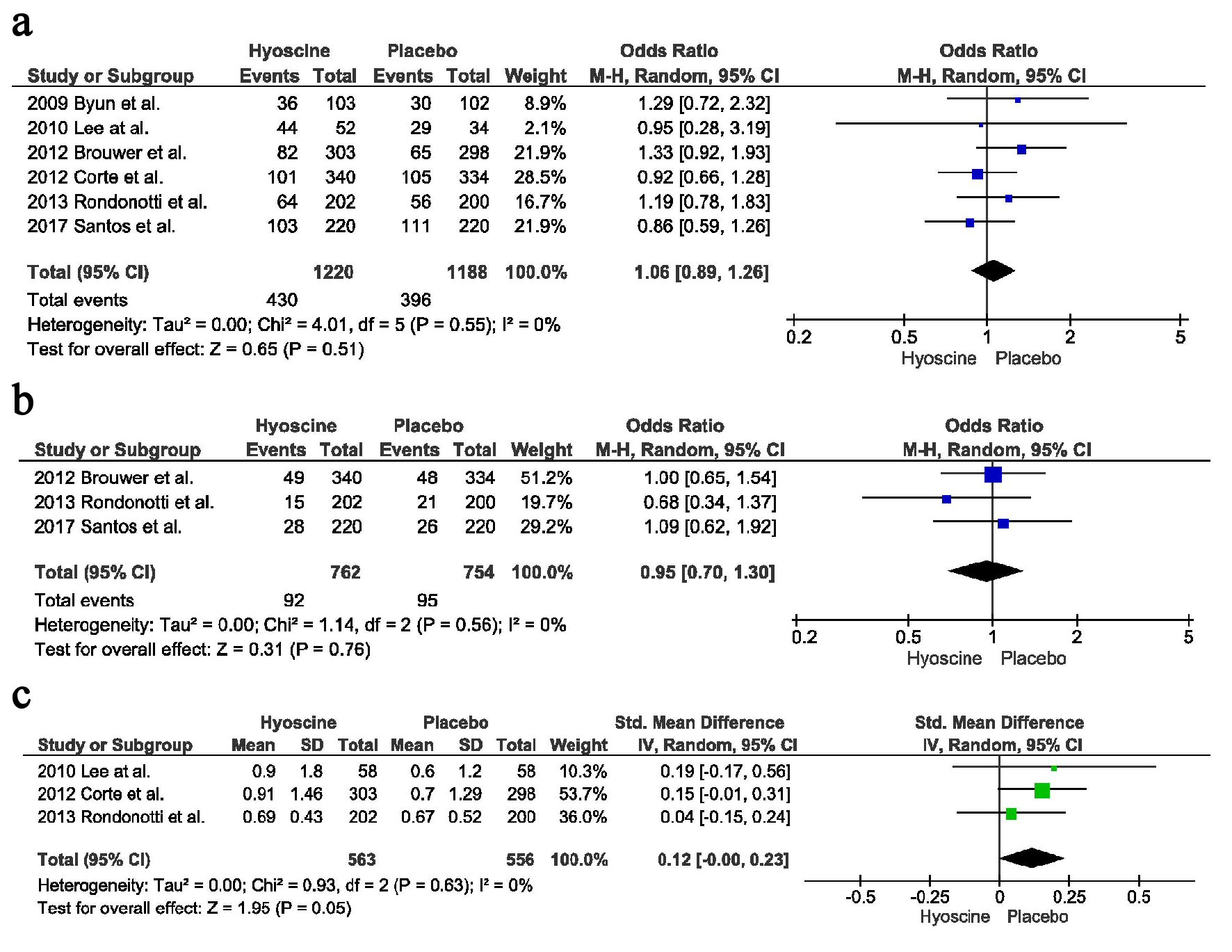
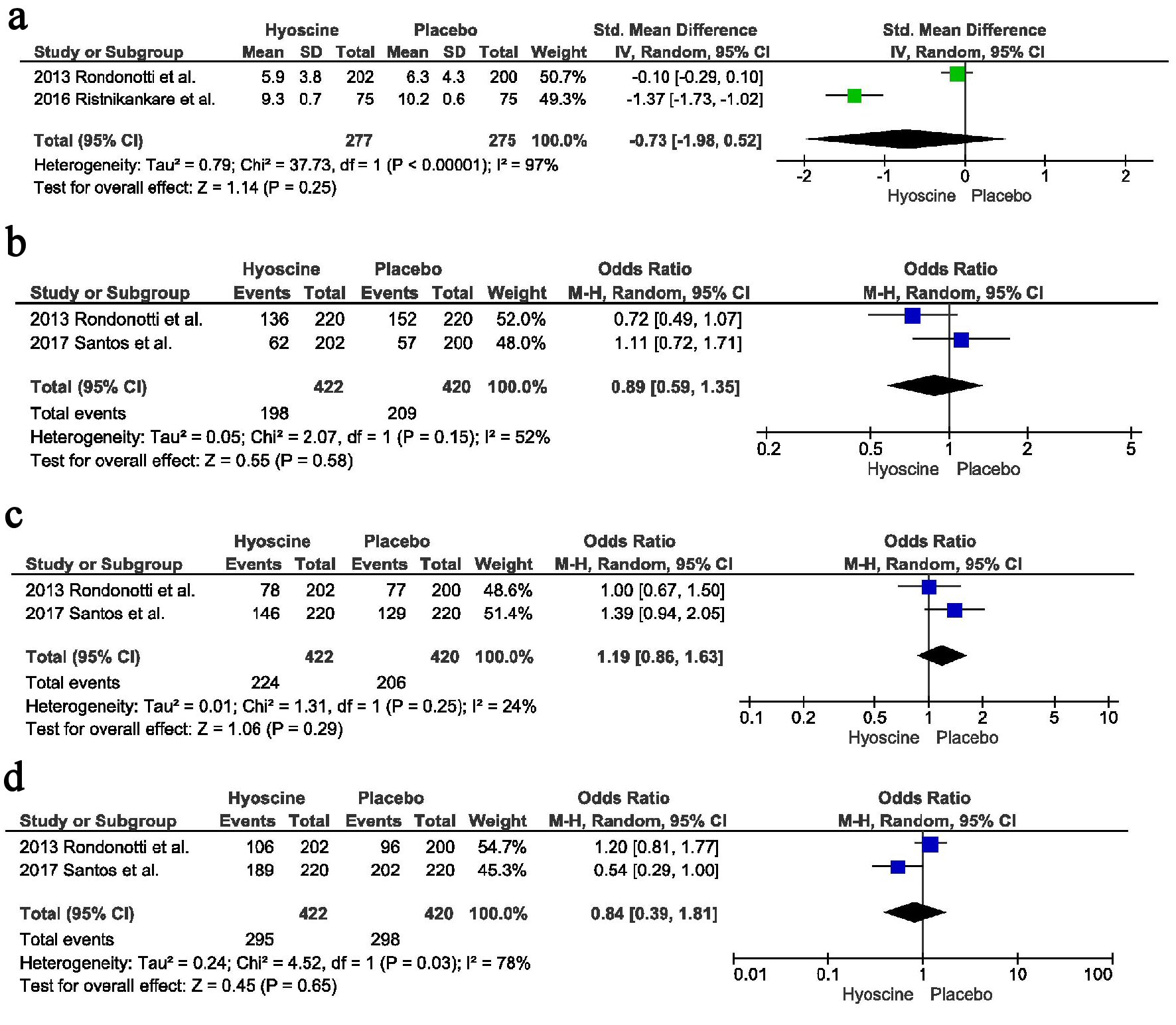
Tables
| *Prior abdominal or pelvic surgery. aDiverticulosis present or >10. bPrior colonoscopy or prior difficult colonoscopy. CRC: colorectal cancer; ABH: altered bowel habits; IBD: irritable bowel disease; NR: not reported; pts: patients. | ||||||||||||||
| Studies | Corte et al [6] | Santos et al [7] | Lee et al [8] | Brouwer et al [11] | Ristikankare et al [9] | Byun et al [12] | Rondonotti et al [10] | |||||||
| Journal | Endoscopy | Clinics | Hepato-Gastroenterology | Gastrointestinal Endoscopy | Scandinavian Journal of Gastroenterology | Gastrointestinal Endoscopy | Digestive and Liver Disease | |||||||
| Design | Prospective RCT | Prospective RCT | Prospective RCT | Prospective RCT | Prospective RCT | Prospective RCT | Prospective RCT | |||||||
| Population | Hyoscine | Placebo | Hyoscine | Placebo | Hyoscine | Placebo | Hyoscine | Placebo | Hyoscine | Placebo | Hyoscine | Placebo | Hyoscine | Placebo |
| Sample size | 303 | 298 | 220 | 220 | 58 | 58 | 340 | 334 | 75 | 75 | 103 | 102 | 202 | 200 |
| Age | 60.6 ± 11.2 | 61.4 ± 10.4 | ≥ 50 (166 pts), < 50 (54 pts) | ≥ 50 (173 pts), < 50 (47 pts) | 59.4 ± 8.5 | 58.4 ± 7.9 | 61.5 | 61.4 | 61.6 ± 8.2 | 59.8 ± 8.9 | NR | NR | 57.3 ± 11.5 | 57.3 ± 13.5 |
| Male no (%) | 162 (53.5) | 157 (52.7) | 73 (33.2) | 69 (31.4) | 27 (46.6) | 23 (39.7) | (45.9) | (52.7) | 37 | 30 | NR | NR | 90 (44.5) | 87 (43.5) |
| BMI (kg/m2) | NR | NR | NR | NR | NR | NR | NR | NR | 26.5 ± 4.5 | 26.1 ± 4.1 | NR | NR | NR | NR |
| Active smoking | NR | NR | NR | NR | 22.4% | 17.2% | NR | NR | 15% | 21% | NR | NR | NR | NR |
| Prior surgery* | 40 (13.4) | 40 (13.2) | NR | NR | NR | NR | NR | NR | 46 | 61 | NR | NR | NR | NR |
| Diverticulosisa | 25 (8.3) | 19 (6.4) | NR | NR | NR | NR | (31.7) | (34.4) | NR | NR | NR | NR | NR | NR |
| Prior colonoscopyb | 26 (8.6) | 23 (7.7) | NR | NR | NR | NR | NR | NR | (39%) | (36%) | NR | NR | NR | NR |
| Indication | ||||||||||||||
| CRC screening | 81 (26.7) | 86 (28.9) | NR | NR | 58 | 58 | NR | NR | NR | NR | NR | NR | 47 (23.2) | 53 (26.5) |
| Polyps surveillance | 58 (19.1) | 52 (17.4) | NR | NR | 0 | 0 | 31 (9.1) | 30 (9) | (11) | (11) | NR | NR | 51 (25.3) | 44 (22) |
| Standard indication | 168 (55.4) | 164 (55) | NR | NR | 0 | 0 | NR | NR | NR | NR | NR | NR | NR | NR |
| Anemia | NR | NR | NR | NR | 0 | 0 | 20 (5.9) | 15 (4.5) | (21) | (39) | NR | NR | 79(39.1) | 81 (40.5) |
| Rectal bleeding | NR | NR | NR | NR | 0 | 0 | 60 (17.6) | 64 (19.2) | NR | NR | ||||
| Abdominal pain | NR | NR | NR | NR | 0 | 0 | 55 (16.2) | 51 (15.3) | (23) | (11) | NR | NR | ||
| Unexplained diarrhea/ABH | NR | NR | NR | NR | 0 | 0 | 31 (9.1) | 34 (10.2) | (21) | (27) | NR | NR | ||
| Screening after resection of CRC | NR | NR | NR | NR | 0 | 0 | 13 (3.8) | 16 (4.8) | NR | NR | NR | NR | NR | NR |
| Family history of CRC | NR | NR | NR | NR | 0 | 0 | 23 (6.8) | 24 (7.2) | NR | NR | NR | NR | 25 (12.4) | 22 (11) |
| IBD dysplasia screening | NR | NR | NR | NR | 0 | 0 | 4 (1.2) | 7 (2.1) | NR | NR | NR | NR | NR | NR |
| Other indication | NR | NR | NR | NR | 0 | 0 | NR | NR | (24) | (13) | NR | NR | NR | NR |
| Study | Corte et al [6] | Santos et al [7] | Lee et al [8] | Brouwer et al [11] | Ristikankare et al [9] | Byun et al [12] | Rondonotti et al [10] |
| Design | Prospective randomized double-blind placebo-controlled trial | Prospective randomized, placebo-controlled trial | Prospective randomized double-blind controlled trial | A prospective, double-blind, placebo-controlled, randomized, clinical trial. | Prospective double-blind, randomized, placebo-controlled, clinical trial | Prospective randomized, double-blinded, placebo-controlled trial | Prospective randomized, double-blind, placebo-controlled trial |
| Country | Australia | Brazil | Korea | Netherlands | Finland | NR | Italy |
| Publication year | 2012 | 2017 | 2010 | 2012 | 2015 | 2009 | 2013 |
| Journal | Endoscopy | Clinics | Hepato-Gastroenterology | Gastrointestinal Endoscopy | Scandinavian Journal of Gastroenterology | Gastrointestinal Endoscopy | Digestive and Liver Disease |
| Enrollment | March 2009 to March 2011 | March to July 2015 | January to June 2008 | January 21 to June 21, 2011 | March 2012 to March 2014 | July 2008 to September 2008 | NR |
| Population | Patients over 40 years old who were scheduled for routine outpatient colonoscopy | CRC screening, surveillance or a clinical suspicion of CRC | Patients between the age 50 and 70 years and had no potential risk factors for CRC | Outpatients aged 30 years or older referred and accepted for colonoscopy | Outpatients scheduled for diagnostic colonoscopy between ages 45 and 75 years | NR | Adult outpatients (18 - 80 years of age) referred for colonoscopy |
| Intervention vs. comparison | Hyoscine vs. placebo | Hyoscine vs. placebo | Hyoscine vs. placebo | Hyoscine vs. placebo | Hyoscine vs. placebo | Hyoscine vs. placebo | Hyoscine vs. placebo |
| Name | Random sequence | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Reporting bias |
|---|---|---|---|---|---|---|
| *Administering nurse not blinded. | ||||||
| Byun et al [12] | Not reported | Not reported | Blinded | Not reported | No | Not reported |
| Unclear | Unclear | Low risk | Unclear | Low Risk | Unclear | |
| Lee et al [8] | Central randomization with computer generated codes | Not reported | Blinded (complete) | Not reported | No | Not reported |
| Low risk | Unclear | Low risk | Unclear | Low Risk | Unclear | |
| Corte et al [6] | Central randomization with computerized algorithm | No | Single arm blinded* | Not reported | No | Not reported |
| Low risk | High risk | Low risk | Unclear | Low risk | Unclear | |
| Brouwer et al [11] | Not reported | Not reported | Not reported | Yes | No | Not reported |
| Unclear | Unclear | Unclear | Low risk | Low risk | Unclear | |
| Rondonotti et al [10] | Central randomization using computer generated list | Randomization list | Single arm blinded* | Not reported | No | Not reported |
| Low risk | Low risk | Low risk | Unclear | Low risk | Unclear | |
| Ristnikankre et al [9] | Block randomization using sealed envelopes | Sealed and coded envelopes | Blinded (complete) | Not reported | No | Not reported |
| Low risk | Low risk | Low risk | Unclear | Low risk | Unclear | |
| Santos et al [7] | Web generated | Sealed envelopes | Blinded | Yes | No | Not reported |
| Low risk | Low risk | Low risk | Low risk | Low risk | Unclear | |